Figure 1. Overall concept of the present study.
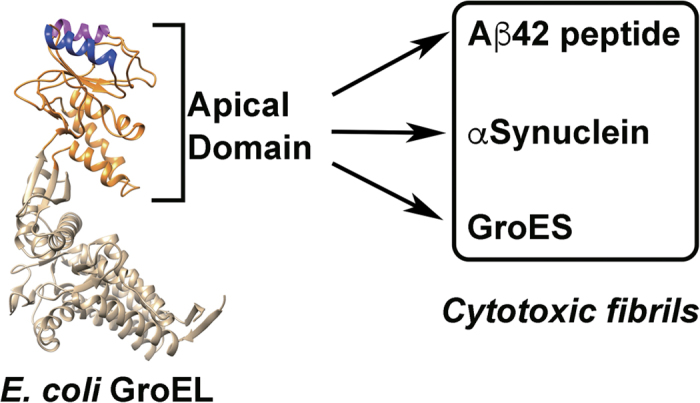
Left, structure of E. coli GroEL subunit derived from PDB 1SVT69. The two helical regions (Helix H, residues Leu234-Ala243 in magenta, and Helix I, residues Gly256-Arg268 in blue70) that form the binding interface for unfolded protein and the co-chaperonin GroES are highlighted. Models were drawn using UCSF Chimera71. The isolated apical domain was used to modulate the fibrillogenesis of three target peptides (Aβ42, α-Synuclein, and GroES). All three polypeptides have either been implicated in the pathogenesis of various diseases, or displayed cytotoxic tendencies in previous experiments50.
