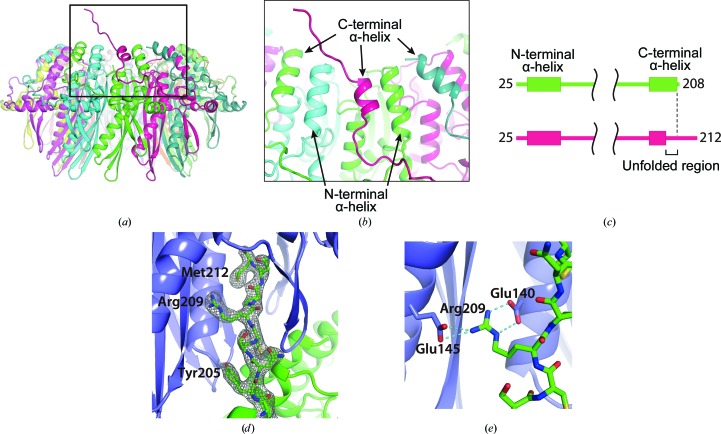
Figure 3.
The C-terminal structure of the mutant RAD521–212. (a) The location of the partially unfolded C-terminal α-helix. The RAD52 monomer containing the unfolded α-helix is coloured magenta. (b) A close-up view of the unfolded α-helix. The C-terminal helices are positioned between the N-terminal helices, such that the N- and C-terminal helices alternate at the surface of the ring near the central channel. The unfolded α-helix is about half a turn shorter than the nearby C-terminal helices. (c) Schematic diagram of the secondary structures of RAD521–212 K102A/K133A/E202A that were visible in the crystal structure. The top diagram (green) corresponds to the RAD52 monomers with a folded C-terminal α-helix. The bottom diagram (magenta) corresponds to the RAD52 monomer containing the unfolded C-terminal α-helix. The RAD52 monomer containing an unfolded α-helix was visible to the C-terminus, whereas the other RAD52 monomers were only visible to amino-acid position 208. (d) The C-terminal region from the mutated RAD521–212 ring (green) is depicted as a stick representation and its 2F o − F c electron-density map (contoured at 1.0σ) is shown. (e) Hydrogen bonding by Arg209 in the C-terminal region. Hydrogen bonds are depicted as dashed lines.