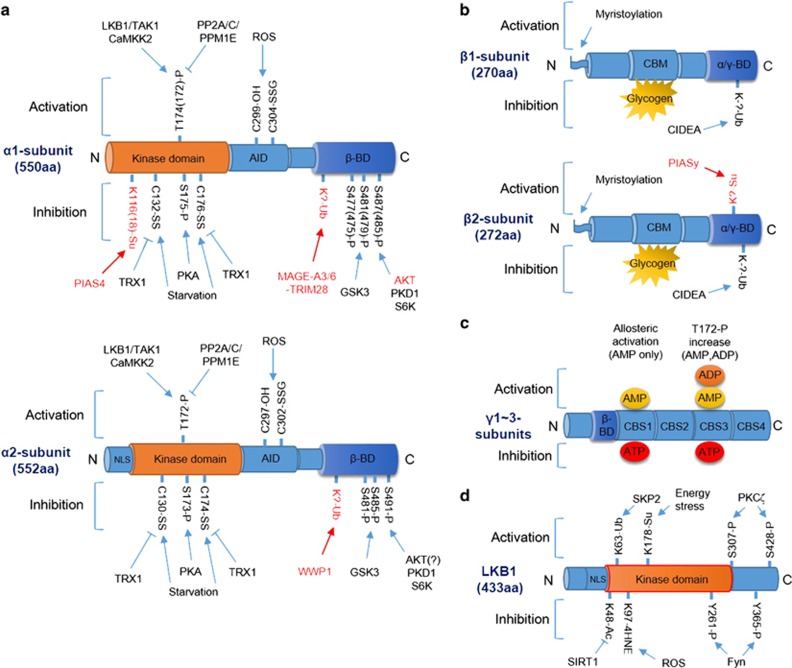
Figure 1.
Molecular regulation of AMPK and LKB1. (a) Modification of the AMPK α1 (top) and α2 (bottom) subunits by phosphorylation/dephosphorylation, ubiquitination, sumoylation and oxidation/reduction. Pathways marked in red indicate α1- or α2-subunit-specific modifications. Numbers of modified amino acids are based on human proteins, and numbers in parenthesis are those reported in the original research (see text for details). (b) Modification of the AMPK β1 (top) and β2 (bottom) subunits by myristoylation, ubiquitination, sumoylation and glycogen binding. Pathways marked in red indicate β1- or β2-subunit-specific modifications (see text for details). (c) Modification of the AMPK γ-subunit by AMP, ADP or ATP binding. Binding of AMP to CBS1 induces allosteric activation, and binding of AMP or ADP to CBS3 induces T172 phosphorylation (see text for details). (d) Modification and regulation of LKB1 by phosphorylation, acetylation, ubiquitination, sumoylation and 4HNE adduction (see text for details). Arrow indicates activation, and bar-headed line indicates inhibition. α/γ-BD, α/γ-subunit-binding domain; AID, autoinhibitory domain; β-BD, β-subunit-binding domain; CBM, carbohydrate-binding module; CBS, cystathionine beta-synthase domain; NLS, nuclear localization signal.