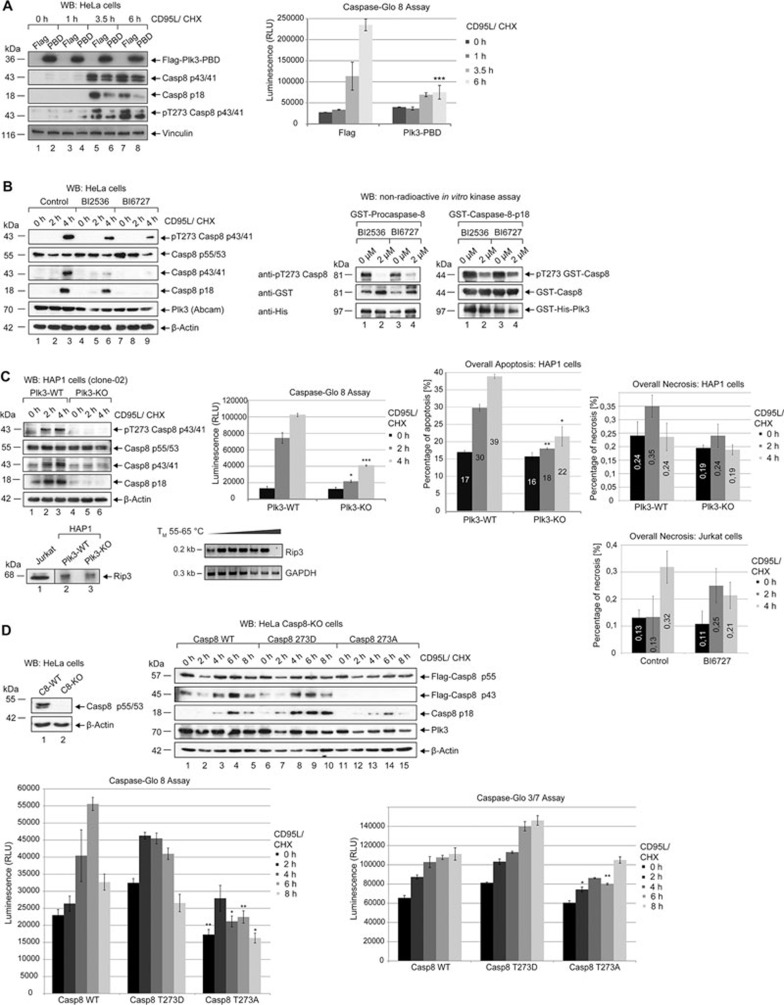
Figure 4.
Inhibiting the function of Plk3 impairs the activation of procaspase-8. (A) HeLa cells were transfected with Flag-fused Plk3-PBD (Flag-Plk3-CT1) or Flag empty vector as a control. 24 h later cells were treated with 50 ng/ml CD95L and CHX for the indicated time periods. The lysates were blotted against Flag, cleaved caspase-8, pT273 caspase-8 and Vinculin (left panel). Caspase-8 activity was determined using a Caspase-Glo 8 assay (right panel). Each bar represents the mean value ± SD (n = 3). The differences between cells that were transfected with Flag or Plk3-PBD were statistically significant by Student's t-test (***P ≤ 0.0005). (B) HeLa cells were pretreated with 2 μM BI2536 or 2 μM BI6727 for 2 h. Untreated cells were used as a control (Control). Subsequently cells were treated with 50 ng/mL CD95L and CHX in the absence (Control) or in the presence of 2 μM BI2536 or BI6727 for the indicated time periods. Lysates were blotted against pT273 caspase-8, caspase-8, cleaved caspase-8, Plk3 and β-actin (left panel). Recombinant GST-fused procaspase-8 and p18 were incubated with recombinant GST-His-fused Plk3 (0.1 μg) in absence or presence of 2 μM BI2536 or BI6727 for 30 min at 37 °C for an in vitro kinase assay. Samples were immunoblotted against pT273 caspase-8, GST and His (right panel). (C) Plk3-WT and Plk3-KO (clone-02) HAP1 cells were treated with CD95L and CHX for the indicated time periods. The lysates were blotted against pT273 caspase-8, caspase-8, cleaved caspase-8 and β-actin (left panel). Caspase-8 activity was determined using a Caspase-Glo 8 assay (middle panel). The overall apoptosis and necrosis were measured by Annexin V/7 AAD FACS analysis (right upper and lower panels). Each bar represents the mean value ± SD (n = 3). The differences between Plk3-WT cells and Plk3-KO cells were statistically significant by Student's t-test (*P ≤ 0.05; **P ≤ 0.005; ***P 0.0005). The protein level of Rip3 in Hap1 and Jurkat cells was monitored by western blot (lower left panel). The mRNA from Hap1 cells was reverse transcribed and Rip3 expression was monitored by PCR with an annealing temperature of 55-65 °C (lower middle panel). GAPDH served as a control. Jurkat cells were treated with 100 ng/mL CD95L and CHX in the absence (Control) or in the presence of 2 μM BI6727 for the indicated time periods. The overall necrosis was measured by Annexin V/7 AAD FACS analysis (lower right panel). Each bar represents the mean value ± SD (n = 3). (D) Lysates of HeLa Casp8-WT and Casp8-KO cells were immunoblotted against caspase-8 and β-actin (left upper panel). In Casp8-KO cells Flag-caspase-8 WT, T273A or T273D mutant was expressed followed by treatment with CD95L and CHX for the indicated time periods. Lysates were blotted against Flag, cleaved caspase-8, Plk3 and β-actin (right upper panel). Caspase-8 and -3/7 activities were determined using Caspase-Glo 8 and 3/7 assays (lower panels). Each bar represents the mean value ± SD (n = 3). The differences between caspase-8 WT and T273A were statistically significant by Student's t-test (*P ≤ 0.05; **P ≤ 0.005).