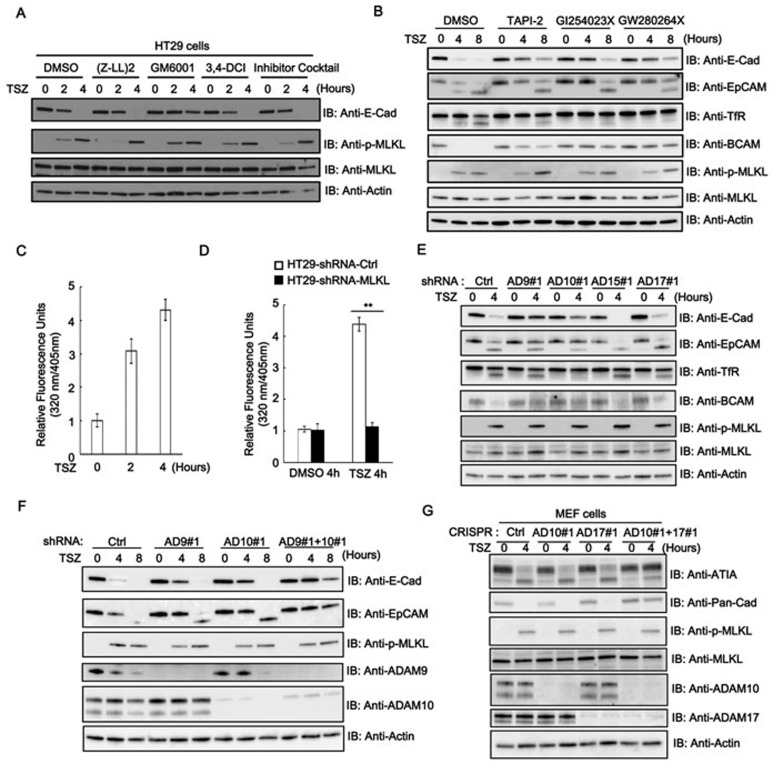
Figure 3.
Activation of ADAM metalloproteinases mediates shedding of cell-surface proteins in necroptosis. (A) HT29 cells were treated with TSZ in the presence of different protinase inhibitors for the indicated time points. Cell lysates were analyzed by immunoblotting as indicated. (B) HT29 cells were treated with TSZ in the presence of ADAM metalloproteinase inhibitors TAPI-2 (10 μM), GI254023X (10 μM), GW280264X (10 μM), or DMSO control (0.1%) at different time points. Cell lysates were analyzed by immunoblotting with the indicated antibodies. (C) Cell-associated metalloprotease activity measured in HT29 cells treated with TSZ at indicated time points. (D) shRNA-Control or shRNA-MLKL HT29 cells were treated with TSZ for 4 h and cell-associated metalloprotease activity was measured. Results shown are averages ± SEM. from three independent experiments. (E) HT29 cells expressing shRNA targeting ADAM9#1, 10#1, 15#1 and 17#1 were treated with TSZ for 4 h. Cell lysates were analyzed by immunoblotting with the indicated antibodies. (F) shRNA control, ADAM9#1, ADAM10#1 or ADAM9#1 and ADAM10#1 HT29 cells were treated with TSZ at different time points and the cell lysates were analyzed by immunoblotting with the indicated antibodies. (G) CRISPR control, ADAM10#1, ADAM17#1 and ADAM10#1/17#1 double-knockout MEF cells were treated with TSZ for 4 h. The cell lysates were analyzed by immunoblotting as indicated. Data shown are representative of three independent experiments. **p< 0.01.