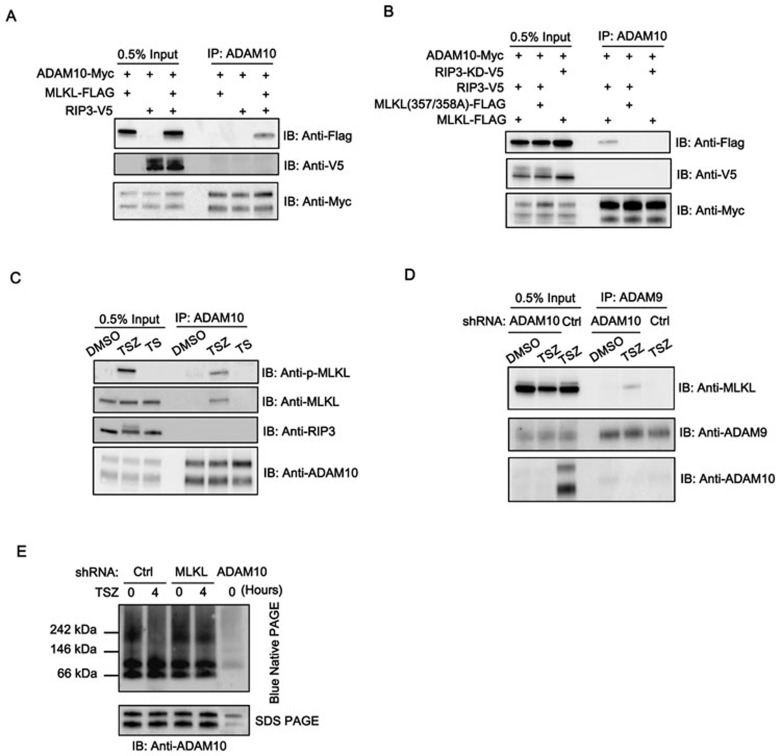
Figure 4.
MLKL forms complex with ADAMs to mediate activation of ADAM metalloproteases. (A) HEK293 cells were transfected with ADAM10-Myc and MLKL-FLAG, RIP3-V5 or MLKL plus RIP3-V5 as indicated. Cell lysates were immunoprecipitated with anti-ADAM10 antibody. The immunoprecipitated complexes were analyzed by immunoblotting as indicated. (B) HEK293 cells were transfected with ADAM10-Myc and other constructs as indicated. Cell lysates were immunoprecipitated with anti-ADAM10 antibody. The immunoprecipitated complexes were analyzed by immunoblotting as indicated. (C) HT29 cells were treated with TSZ or TS for 4 h. Cell lysates were immunoprecipitated with anti-ADAM10 antibody. The immunoprecipitated complexes were analyzed by immunoblotting as indicated. (D) shRNA-Control or shRNA-MLKL HT29 cells were treated with TSZ for 4 h. Cell lysates were immunoprecipitated with anti-ADAM9 antibody. (E) shRNA-control or -MLKL HT29 cells were treated with TSZ for 4 h. The cells were lysed under native condition and analyzed by blue native PAGE (upper panel) or SDS-PAGE (lower panel) with anti-ADAM10 immunoblotting. The cell lysate from ADAM10 knockdown cells was used as negative control for ADAM10 antibody immunoblotting. Data shown are representative of three independent experiments.