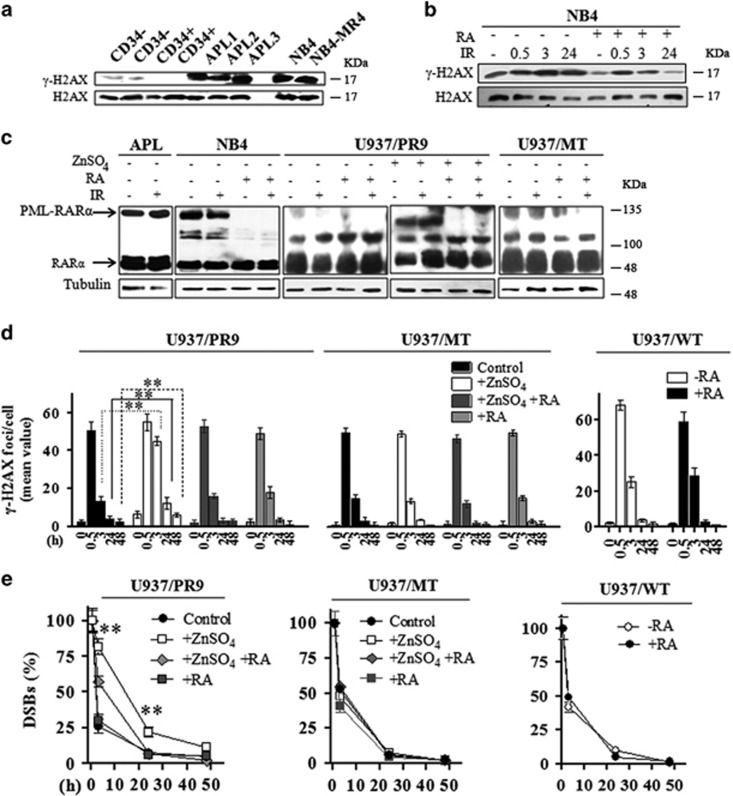
Figure 2.
(a) Representative immunoblot analysis of H2AX and H2AX phosphorylation at the Ser139 residue in untreated human CD34− and CD34+ cells isolated from the peripheral blood of normal donors, in three APL patients, in NB4 and NB4-MR4 cells. (b) Representative immunoblot analysis of H2AX phosphorylation in NB4 cells treated or not with 1 μM RA for 72 h and then irradiated with 1 Gy of X-rays and lysed after 0.5, 3, and 24 h. (c) Representative immunoblot analysis of RARα and PML-RARα expression levels in APL blasts, NB4, U937/PR9, and U937/MT cells exposed to IR and lysed after 0.5 h; before irradiation, NB4 cells were treated or not with 1 μM RA for 72 h, whereas U937/PR9 cells were treated or not with 100 μM ZnSO4 for 8 h and then with 1 μM RA for 72 h, as indicated; filters were probed with anti-RARα antibody, and tubulin was used as loading control. (d) Quantification of the mean number of γ-H2AX foci/cell and (e) analysis of the rejoining capability measured as percentage of DSBs in U937/PR9, U937/MT, and U937/WT cells treated or not with 100 μM ZnSO4 for 8 h and/or with 1 μM RA for 72 h, as indicated; cells were then exposed to IR and fixed at the indicated times. Mean values were derived from the analysis of 100 cells in three independent experiments±S.D. For DSBs, the mean number of γ-H2AX foci/cell measurable at 0.5 h after IR was taken as 100%. **P<0.01. For U937/PR9 DSB rejoining graph; **indicates significant differences of ZnSO4-treated U937/PR9 cells with respect to U937/PR9 control cells. Confocal analysis was performed using the LCS Leica confocal microscope (Leica Microsystems)