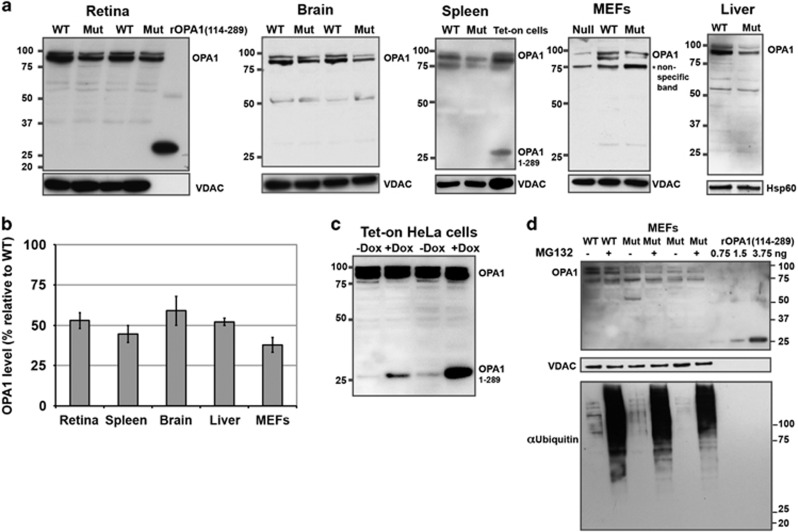
Figure 3.
Mutated Opa1 is not expressed at detectable levels in Opa1Q285STOP mice. (a) Samples of indicated tissues derived from the Opa1Q285STOP mice were probed for the presence of Opa11–285 protein by western blot analysis using Opa1 antisera generated against an N-terminal Opa1 polypeptide. As a positive control for the specific antisera reactivity, samples of HeLa cells with doxycycline-inducible expression of Opa11–289 (also shown in panel c) or the recombinant Opa1 polypeptide (rOpa1114–289) were loaded on the gels, where indicated. As a negative control for antibody crossreactivity, a sample of Opa1-null cells was added, where indicated. Samples were loaded at 30–50 μg per lane. Endogenous truncated Opa1 was not detected in any of the samples tested (including isolated liver mitochondria), whereas WT Opa1 and inducibly expressed Opa11–289 were readily detected by the Opa1 antisera. (b) Quantification of Opa1 protein level confirms its ~50% reduction in all mutant samples. (c) Doxycycline-induced Opa11–289 expression in HeLa cells one day (second lane) or 2 days (last lane) after the addition of doxycycline. Whole-cell lysates were loaded at 30 μg per lane. (d) Proteasomal inhibition by MG132 does not lead to accumulation of endogenous Opa11–285. MEFs were pretreated with 2 μM MG132 for 24 h before cell lysate preparation. (Last three lanes on the blot contained the recombinant Opa1 polypeptide loaded at indicated amounts.) The membrane was reprobed with ubiquitin antibody to confirm accumulation of ubiquinated proteins and VDAC antibody for a loading control