Table 1.
Reaction Optimization[a].
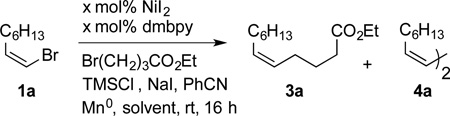 | |||||
|---|---|---|---|---|---|
| Entry | x | NaI [mol %] |
Solvent | Yield 3a [%][b] |
Yield 4a [%][b] |
| 1[c] | 5.0 | - | DMA | 12 | 27 |
| 2 | 5.0 | - | DMA | 15 | 19 |
| 3 | 5.0 | 25 | DMA | 36 | 14 |
| 4 | 5.0 | 50 | DMA | 57 | 15 |
| 5 | 1.0 | 50 | DMA | 62 | 10 |
| 6[d] | 1.0 | 50 | DMA | 71 | 7 |
| 7[d] | 1.0 | 50 | DMF | 61 | 11 |
| 8[d] | 1.0 | 50 | DMPU | 88 (80)[e] | 4 |
| 9[f] | 5.0 | 25 | DMPU | 44 | n.d. |
Reactions performed on 0.5 mmol scale (0.25 m) using a 1:1 ratio of starting materials, benzonitrile (5 mol%), chlorotrimethylsilane (20 mol%), manganese metal (2 equiv).
Determined by GC using dodecane as an internal standard. 4a yields are uncorrected.
60 °C, 2h.
(bpy)NiI2 used as a precatalyst.
Isolated yield.
As in reference [4b].
n.d. = not determined, dmbpy = 4,4’-dimethoxy-2,2’-bipyridine, DMA = N,N-dimethylacetamide, DMF = N,N-dimethylformamide, DMPU = N,N'-dimethylpropyleneurea.
