ABSTRACT
Adult Neural Stem Cells (aNSCs) generate new neurons that integrate into the pre-existing networks in specific locations of the Vertebrate brain. Moreover, aNSCs contribute with new neurons to brain regeneration in some non-mammalian Vertebrates. The similarities and the differences in the cellular and molecular processes governing neurogenesis in the intact and regenerating brain are still to be assessed. Toward this end, we recently established a protocol for non-invasive imaging of aNSC behavior in their niche in vivo in the adult intact and regenerating zebrafish telencephalon. We observed different modes of aNSC division in the intact brain and a novel mode of neurogenesis by direct conversion, which contributes to stem cell depletion with age. After injury, the generation of neurons is increased both by the activation of additional aNSCs and a shift in the division mode of aNSCs, thereby contributing to the successful neuronal regeneration. The cellular behavior we observed opens new questions regarding long-term aNSC maintenance in homeostasis and in regeneration. In this commentary we discuss our data and new questions arising in the context of aNSC behavior, not only in zebrafish but also in other species, including mammals.
KEYWORDS: cell division, cell fate conversion, in vivo imaging, neural stem cell, neurogenesis, regeneration
In the adult vertebrate brain, new neurons are produced1,4,23,39,59 and turned over38,44 throughout the animal's life, with an age-dependent decline14,15,21,33,56 These neurons differentiate from neural stem cells (NSCs) that reside in specialized niches within the brain1,17,29,54 The existence of adult/ neonatal NSCs, also in the human brain,22,23,59 raised hope for their use in regenerative therapies for neurodegenerative diseases or brain injuries. Indeed, adult NSCs (aNSCs) provide new neurons engaged in the repair of the injured brain in regeneration-competent species, such as zebrafish8,10,11,36,37,58,65 Although the aNSCs contribute with new, mature neurons in some regeneration-competent species, the first attempts to utilize the endogenous aNSCs for repair in the mammalian brain largely failed,7,58,61,62 probably due to the lack of understanding of basic aNSC biology. Moreover, it also remains unclear to which extent the regeneration of the injured brain requires changes in the behavior of aNSCs compared to the intact brain in order to complete the regeneration process. For example, repair of the injured cerebral cortex would require the generation of pyramidal neurons, a cell type never produced by the aNSCs in the intact brain.46 Therefore, it is crucial to compare the cellular behavior at the single stem cell level in the intact and injured brain of regeneration-incompetent and regeneration-competent species. The first approaches to understand the cellular behavior of single aNSCs in the neurogenic zones of the intact mammalian brain based on clonal analysis13,15 revealed the fast consumption of a single aNSC that produces a heterogeneous neuronal output. However, different cellular processes such as cell death, selective proliferation and terminal differentiation could yield into the described features of adult mammalian neurogenesis. The methods used in these studies could not provide complete information on the continuous behavior/dynamics of aNSCs in their intact niche, highlighting the need for complementary methods that allow repeated observation of the same aNSCs in their biological environment.
In the mammalian brain the NSC niches are rare (Sub-ependymal zone (SEZ), dentate gyrus (DG) and hypothalamus) and are located several hundreds of µm away from the brain surface3,39,52 rendering them inaccessible for direct observations. In contrast, the neurogenic niches in the adult zebrafish are widespread throughout the whole brain and accessible for live imaging, particularly in the dorsal telencephalon, due to its location at the outer surface of the brain (Fig. 1a).1,29 The privileged location of aNCSs in the dorsal zebrafish telencephalon, and the exceptional regenerative potential of this brain region, makes the zebrafish pallium an attractive system to pursue in vivo imaging experiments.
Figure 1.
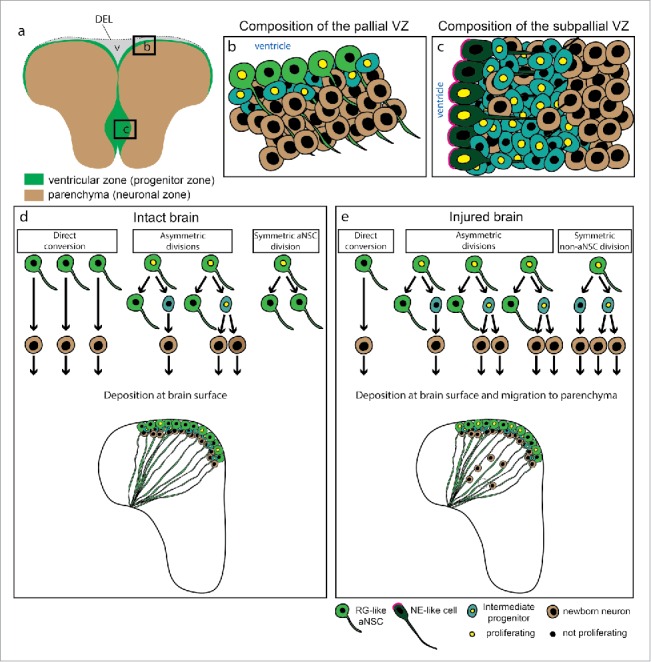
Neurogenic niches in the adult zebrafish telencephalon and behavior of aNSCs in the pallium. (a) Representation of a coronal section through the adult zebrafish telencephalon illustrating the ventricular zone (green), containing aNSCs and progenitors, and the parenchyma (brown), mostly composed of neurons. The two hemispheres are linked by a dorsal ependymal lining (DEL) that closes the ventricle (v). (b) and (c) Scheme of the dorsal (b) and ventral (c) ventricular zones, composed of different progenitor types: RG-like aNSCs, non-glial intermediate progenitors and neuro-epithelial-like cells. (d) and (e) Modes of cell division and neurogenesis in the intact (d) and injured (e) zebrafish telencephalon, assessed by live imaging.10 In the intact brain (d) the newborn neurons are deposited immediately adjacent to the progenitor cells. After injury (e) there is an increase in proliferating aNSCs at the VZ, a change in their mode of cell division and the migration of progeny to the injured parenchyma, where the newborn neurons contribute to tissue regeneration. The dashed circle marks the region where the lesion was. Abbreviations: DEL-dorsal ependymal lining; NE-neurepithelial; RG-radial glia; VZ-ventricular zone.
Live in vivo imaging allows for the integrative view on the changes in behavior of the single aNSCs and their progeny in the regenerating and intact zebrafish brain. This commentary will discuss the behavior of the aNSCs in the zebrafish telencephalon in both conditions and its possible implications for the processes of aging and regeneration.
Output of the adult neural stem cells in the intact and injured brain
The neurogenic niche in the adult zebrafish pallium, accessible for live imaging, contains radial glia-like aNSCs (Fig. 1b) with their cell bodies lining the ventricular wall. Radial processes of aNSCs span the brain parenchyma to contact the basement membrane.10,24,42 The morphology and the antigen profile of aNSCs in the zebrafish pallium are reminiscent of radial glial (RG) cells in the developing mouse telencephalon.24,27,28,30,42 The aNSCs in the zebrafish pallium do not only share the morphological and immunohistochemical characteristics with the mammalian RG cells in the developing brain, but also have the capacity to generate new neurons. However, in contrast to the new neurons produced in the developing mammalian cerebral cortex,6,46 the new neurons produced by the pallial aNSCs in the intact brain of zebrafish do not migrate away from the stem cell zone and are instead deposited directly below the progenitor zone (Fig. 1d)1,29,42,53 These new neurons are intermingled with fast dividing progenitors (Fig. 1b) that do not have stem cell characteristics (intermediate progenitors (IPs) or non-glial progenitors).24,42,53 Traumatic brain injury induces a specific program in the aNSCs and intermediate progenitors resulting in the production of new neurons necessary for regeneration.35 In contrast to the intact brain, these newborn neurons migrate larger distances to repopulate the damaged brain areas (Fig. 1e).10,36 Importantly, the small stab wound injury induces the restorative neurogenesis without an impact on the ongoing neurogenesis present in the intact brain.11 This indeed raises the question of the origin of the new neurons engaged in the repair process. Moreover, it remains unclear to which extent the cellular processes underlying restorative neurogenesis recapitulate those sustaining the normal generation of adult-born neurons.
To address these questions, we repetitively imaged aNSCs in the Tg(gfap::GFP)mir2001 transgenic fish line that expresses GFP in all aNSCs. To reliably re-identify aNSCs in different imaging sessions, we sparsely labeled a small number of them by electroporation of a reporter (TdTomatomem) encoding plasmid. As the ubiquitous cytomagalovirus (CMV) promoter drives the expression of the reporter, we could follow not only aNSCs, but also their progeny that lost the radial morphology and glial marker expression.10 Our results confirmed previous observations that in the intact brain aNSCs are mostly quiescent and only a small proportion of aNSCs divide or change their identity to generate progeny (aNSCs activation) at any discrete time point. Both live imaging10,19 and clonal analysis10,53 suggest that aNSCs predominantly generate neurons upon their activation. In addition, we could observe the generation of gfap::GFP-positive glial cells indistinguishable from the mother cell10 indicative of self-renewal. Indeed, all aNSC divisions in the intact brain generated at least one cell with radial morphology and also expressing gfap::GFP. Whether some of these glial cells are already a differentiated cell type (possibly homologous to the ependymal cells in the mammalian brain) or a quiescent stem cell is not clear yet. However, virtually all GFAP-positive glial cells in the zebrafish pallium can be recruited into the cell cycle upon Notch inhibition,5 strongly suggesting that at least some of the newly generated gfap::GFP-positive cells with radial morphology are quiescent aNSCs.10 Longer imaging periods would be required to tackle this question in a comprehensive manner.
The generation of oligodendrocytes from aNSCs in the zebrafish pallium has not been directly assessed. However, aNSC-derived cells mostly express either GFAP or neuronal lineage markers (Sox2 and HuCD) (unpublished data), accentuating the idea that only neurons and aNSCs/ependyma are generated from NSCs in this brain region. This is indeed very similar to the adult mouse DG, where aNSCs do not generate oligodendrocytes.13 Oligodendrocytes are generated in this region exclusively by neural/glial antigen2 (NG2)-positive progenitors32 In the adult mouse SEZ oligodendrocytes are also generated, but the neuronal and oligodendroglial lineages are separated in two independent populations of progenitors.15,48
Although the predominantly neuronal output appears to be a hallmark of aNSCs in the Vertebrate brain, the cohort size produced by a single aNSC differs greatly between species and analyzed areas (Fig. 2). Despite the difference in clone size, the output of a single aNSC seems to be defined by the number of divisions of intermediate progenitors. In the zebrafish pallium, these progenitors divide once or twice before terminally differentiating into neurons10,53 Interestingly, this low degree of lineage amplification in the zebrafish pallium is reminiscent of the behavior of progenitors in the adult mouse DG,13,21,40 supporting the suggested homology between the two regions (Fig. 2).25,51,64 Also in the developing rodent dorsal telencephalon, basal/intermediate progenitors divide few times before generating neurons (Fig. 2a).26,44,46 However, this situation contrasts with the mouse ventral telencephalon, in which multiple rounds of division of progenitors greatly amplify the neuronal output (Fig. 2a)49 Similarly, in the SEZ niche a high degree of amplification occurs at the transit amplifying progenitor (3–4 divisions) and neuroblast (1–2 divisions) level15,50 Moreover, aNSCs also divide asymmetrically several times each 2–3 weeks to produce a larger neuronal output containing several cohorts (Fig. 2b).15 Unlike in the pallium, the neurogenic niche in the zebrafish subpallium appears more similar to the mouse SEZ (Fig. 1c), as there are proportionally more IPs24,42 and some of these also migrate to the olfactory bulb.34 Interestingly, in the zebrafish subpallium the aNSCs exhibit low levels or no expression of glial markers and, because they express nestin and the tight junction component zona occludens 1 (ZO-1),24 they resemble neuroepithelial cells (Fig. 1c). The mixture of radial and tangential migration of the NSC progeny in this region makes a clonal analysis challenging. Thus, the behavior of subpallial aNSC in the zebrafish at the single cell level remains to be assessed.
Figure 2.
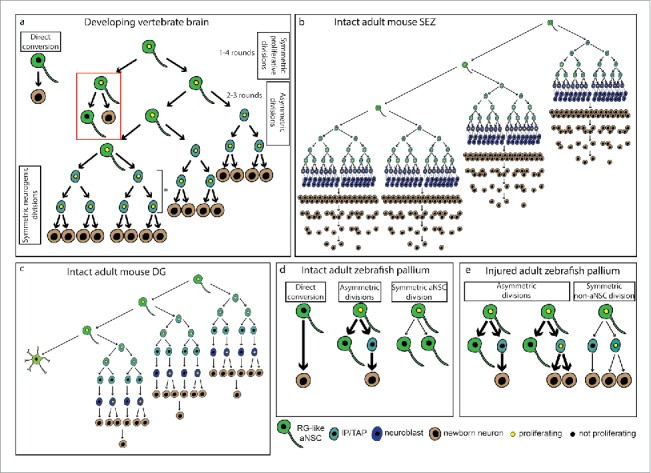
Neuronal output of single NSCs. (a) Different modes of neuron generation in the developing brain. In the zebrafish hindbrain and Xenopus optic tectum, some NSCs directly convert into neurons (direct conversion), depleting themselves. At early embryonic stages in the zebrafish and mouse telencephalon, NSCs proliferate (1–4 rounds in the mouse) to increase their number (symmetric proliferative divisions).18,26 As development advances, NSCs divide asymmetrically to give rise to another NSC and a neuron directly (direct neurogenesis, red box) or to a NSC and an intermediate progenitor, that amplifies the neuron production (indirect neurogenesis). In the mouse cortex, NSCs can undergo 2–3 rounds of asymmetric division.26 The degree of amplification via intermediate progenitors differs in the cortex and the ventral telencephalon of the mouse (asterisk), since cortical IPs divide once or twice before generating neurons, whereas in the lateral ganglionic eminence they divide several times. At the end of neurogenesis, most NSCs undergo a terminal division (symmetric neurogenic divisions). (b) NSC lineage in the adult mouse SEZ. Adult NSCs have a limited self-renewal capacity, and generate neurons via transit amplifying progenitors (TAPs) and neuroblasts. Despite the high degree of amplification, many neurons die and only a few manage to survive and integrate in the olfactory bulb circuits.15 (c) NSC lineage in the adult mouse DG. In this region aNSCs undergo a few rounds of asymmetric divisions to generate neurons via intermediate progenitors, after which they terminally differentiate into astrocytes. Compared to the SEZ, there is a lower amplification at the intermediate progenitor level.13,21 (d) Modes of neurogenesis and division in the intact adult zebrafish pallium. Neurogenesis occurs either through direct conversion, depleting the NSC, or asymmetric division, maintaining the aNSC. Besides the self-renewing asymmetric division, NSCs also undergo symmetric amplifying divisions (symmetric aNSC division). The thickness of the arrows represents the relative frequency of each behavior. (e) Modes of neurogenesis and division in the injured adult zebrafish pallium. After injury, aNSCs divide not only asymmetrically, as in the intact brain, but also symmetrically to generate two intermediate progenitors, increasing the neuronal output. The thickness of the arrows represents the relative frequency of each behavior. Abbreviations: aNSC-adult neural stem cell; DG-dentate gyrus; IP-intermediate progenitor; RG-radial glia; SEZ-sub-ependymal zone; TAP-transit amplifying progenitor.
The broad spectrum of the single aNSC behaviors leading to the production of differently sized neuronal cohorts prompted us to search also for the mechanisms enlarging the neuronal output during regeneration, that allow the replacement of the lost neurons without an obvious impact on the ongoing neurogenesis.10,11 Several cellular mechanisms such as increased aNSC proliferation, proliferation of the non-glial progenitors, decreased cell death etc. could account for this net increase in neuronal production. Indeed, both aNSCs and non-glial progenitors increase their proliferation after brain injury11,36,43 However, we could not observe multiple divisions of a single aNSC using live imaging in vivo, but rather increased recruitment of quiescent aNSCs.10 Interestingly, we observed that some of the aNSCs dividing after injury did not self-renew, but rather exhausted themselves by a symmetric division generating two non-glial progenitors.10 This mode of division, not present in the intact brain, might constitute an advantage after injury, since it generates a larger neuronal output from a single aNSC compared to an asymmetric division. On the other hand, it also leads to the depletion of stem cells. Curiously, enhanced aNSC depletion has also been observed in the DG of a mouse model of neuronal hyperactivity, suggesting some conservation of aNSC reaction to challenge in different Vertebrate species.57 This NSC exhaustion phenomenon would predict a decreased capacity of the zebrafish brain to regenerate damages induced by the repetition of the insult. Alternatively, aNSCs in the zebrafish might be heterogeneous and could contain more primitive, undifferentiated cells16 with the capacity to repopulate aNSCs depleted after injury and enable proper regeneration even after multiple injuries. Indeed, in the zebrafish caudal fin amputation paradigm, progenitor cells completely reconstitute the entire damaged tissue without losing efficiency even after repeated amputations.9,55 Therefore, it would be important to address if aNSCs have the capacity to repopulate the neurogenic zone and allow tissue restoration even after multiple insults or if the different organs have different regeneration capacity depending on the characteristics of the somatic stem cells residing within the given organ.
Direct conversion
An important novel feature of adult neurogenesis in the zebrafish telencephalon uncovered by our in vivo imaging was the direct conversion of a considerable proportion of aNSCs (50 % of all aNSCs generating neurons) into a neuron without any cell division.10 Interestingly, this type of neurogenesis is also described in developing brains. In fact, single progenitors labeled at the neural rod stage directly convert into neurons without cell division in the developing zebrafish hindbrain.41 Also in Xenopus laevis, time-lapse imaging demonstrated that the majority of RG cells in the developing optic tectum directly differentiate into neurons.12 As direct conversion had never been observed in the adult brain, this could either mean that aNSCs in the zebrafish telencephalon possess this unique capacity, or that direct conversion has not yet been detected in the adult brain of other species due to the lack of suitable technical approaches such as live imaging.
At present, we cannot elucidate if the population of aNSCs that directly convert to neurons is a specific population or if these cells also have the capacity to divide. In fact, both in the zebrafish2,18 and in the mouse embryo,31,44,46 dividing RG cells generate neurons directly without going through an intermediate progenitor state (Fig. 2a, direct neurogenesis). In our study however, we followed aNSCs for at least 2 weeks before they directly converted into neurons, suggesting that these cells either have an extremely long cell cycle or do not need to divide at all to generate neurons.
Notably, after injury aNSCs divide more and change their division mode to produce large cohorts of neuronal progeny needed in these hostile conditions. In contrast, direct conversion would allow slow, but constant addition of new neurons to the slowly growing adult. It is still to be addressed if the aNSCs undergoing direct conversion in the intact brain would become activated after injury and divide in a symmetric neurogenic manner (a mode of aNSCs division that we observe only in the injured brain) to produce a larger neuronal output and deplete themselves after injury. Alternatively, direct conversion might be actively blocked but these aNSCs would be kept at the VZ for the slow neuronal production after the brain is regenerated.
Stem cell depletion-a common feature of the vertebrate neurogenesis
In the adult zebrafish pallium, new neurons are added either by asymmetric division of aNSCs, thus maintaining the stem cell pool, or by direct conversion depleting the stem cell pool. Our live imaging showed that the proportion of aNSCs that directly convert to neurons and deplete themselves (17%) is considerably higher than the proportion of aNSCs dividing symmetrically to amplify the stem cell population (1%) in the intact brain.10 This finding implies a gradual consumption of aNSCs with age. Indeed the number of proliferating aNSCs was decreasing in 6 and 10 months old animals compared to 3 months old animals,10 which correlates with the previously described decrease in neurogenesis in the aged zebrafish pallium.20 These data therefore would support the hypothesis that aNSC depletion is the cellular basis for the age-dependent decline of neurogenesis in zebrafish. However, Edelmann et al observed that the total number of gfap::GFP -positive glia (regardless of their proliferative status) does not decrease with aging.20 The cellular mechanisms that maintain the ependymoglia in the adult brain remain to be investigated, but one possible explanation would be that the modes of stem cell division/behavior could be changed toward the gliogenic/ependymoglial fate in aging animals. In our study we used 2–3 months-old animals and followed single cells for one month but it is possible that in older animals there is a prevalence of symmetric aNSCs divisions that would replenish the pool of ependymoglia. More in vivo imaging of single aNSCs at these later stages would be needed to clarify these issues. Importantly, in the mouse neurogenic niches, SEZ and dentate gyrus, there is a limited number of self-renewing divisions of aNSCs followed by terminal differentiation into the neuronal or astrocytic lineage.13,15,21 Therefore the exhaustion of aNSCs in Vertebrates is a common trait, possibly including the glial fate as the final differentiation step.
Future directions
In summary, the in vivo imaging we established allowed for the first time the visualization of aNSCs in their natural niche in a vertebrate model, not only in physiological conditions but also after brain damage. Consequently, several features of the behavior of individual aNSCs were revealed at the single cell level, at an extent that could not be assessed by previous population-based studies. The knowledge acquired with the observation of NSC behavior in the zebrafish brain may serve as a basis to conduct studies on modulating NSC activity in the diseased brain. For the application of these findings in mammalian disease models it will be important to compare the NSC behavior in zebrafish and mammals.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We want to thank Magdalena Götz, David Petrik, Filippo Calzolari, Sven Falk and Rosario Sanchez-Gonzalez for critical reading of the manuscript.
Funding
We also gratefully acknowledge funding to JN from the German Research foundation (DFG) by the SFB 870 and SPP “Integrative Analysis of Olfaction” and to JSB from the Fundação para a Ciência e Tecnologia, Portugal (FCT).
References
- [1].Adolf B, Chapouton P, Lam CS, Topp S, Tannhauser B, Strahle U, Gotz M, Bally-Cuif L.. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol 2006; 295:278-93; PMID:16828638 [DOI] [PubMed] [Google Scholar]
- [2].Alexandre PR, Alexander M, Barker D, Blanc E, Clarke JDW.. Neurons derive from the more apical daughter in asymmetric divisions in the zebrafish neural tube. Nat Neurosci 2010; 13:673-9; PMID:20453852; http://dx.doi.org/ 10.1038/nn.2547 [DOI] [PubMed] [Google Scholar]
- [3].Altman J, Das GD.. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comparative Neurol 1965a; 124:319-35; http://dx.doi.org/ 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- [4].Altman J, Das GD.. Post-natal origin of microneurones in the rat brain. Nature 1965b; 207:953-6; http://dx.doi.org/ 10.1038/207953a0 [DOI] [PubMed] [Google Scholar]
- [5].Alunni A, Krecsmarik M, Bosco A, Galant S, Pan L, Moens CB, Bally-Cuif L.. Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development 2013; 140:3335-47; PMID:23863484; http://dx.doi.org/ 10.1242/dev.095018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Angevine JB, Sidman RL.. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 1961; 192:766-8; PMID:17533671; http://dx.doi.org/ 10.1038/192766b0 [DOI] [PubMed] [Google Scholar]
- [7].Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O.. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002; 8:963-70; PMID:12161747; http://dx.doi.org/ 10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- [8].Ayari B, Elhachimi KH, Yanicostas C, Landoulsi A, Soussi-Yanicostas N.. Prokineticin 2 expression is associated with neural repair of injured adult zebrafish telencephalon. J Neurotrauma 2010; 27:959-72; PMID:20102264; http://dx.doi.org/ 10.1089/neu.2009.0972 [DOI] [PubMed] [Google Scholar]
- [9].Azevedo AS, Grotek B, Jacinto A, Weidinger G, Saúde L.. The Regenerative capacity of the zebrafish caudal fin is not affected by repeated amputations. PLoS One 2011; 6:e22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barbosa JS, Sanchez-Gonzalez R, Di Giaimo R, Baumgart EV, Theis FJ, Götz M, Ninkovic J.. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science 2015; 348:789-93; PMID:25977550; http://dx.doi.org/ 10.1126/science.aaa2729 [DOI] [PubMed] [Google Scholar]
- [11].Baumgart EV, Barbosa JS, Bally-Cuif L, Gotz M, Ninkovic J.. Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 2012; 60:343-57; PMID:22105794; http://dx.doi.org/ 10.1002/glia.22269 [DOI] [PubMed] [Google Scholar]
- [12].Bestman JE, Lee-Osbourne J, Cline HT.. In vivo time-lapse imaging of cell proliferation and differentiation in the optic tectum of Xenopus laevis tadpoles. J Comparative Neurol 2012; 520:401-33; PMID:22113462; http://dx.doi.org/ 10.1002/cne.22795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H.. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011; 145:1142-55; PMID:21664664; http://dx.doi.org/ 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bouab M, Paliouras GN, Aumont A, Forest-Bérard K, Fernandes KJL.. Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neurosci 2011; 173:135-49; PMID:21094223; http://dx.doi.org/ 10.1016/j.neuroscience.2010.11.032 [DOI] [PubMed] [Google Scholar]
- [15].Calzolari F, Michel J, Baumgart EV, Theis F, Gotz M, Ninkovic J.. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat Neurosci 2015; 18:490-2; PMID:25730673; http://dx.doi.org/ 10.1038/nn.3963 [DOI] [PubMed] [Google Scholar]
- [16].Dirian L, Galant S, Coolen M, Chen W, Bedu S, Houart C, Bally-Cuif L, Foucher I.. Spatial regionalization and heterochrony in the formation of adult pallial neural stem cells. Dev Cell 2014; 30(2):123-36; PMID:25017692 [DOI] [PubMed] [Google Scholar]
- [17].Doetsch R, Caille I, Lim D, Garcia-Verdugo J, Alvarez-Buylla A.. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703-16; PMID:10380923; http://dx.doi.org/ 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- [18].Dong Z, Yang N, Yeo SY, Chitnis A, Guo S.. Intralineage directional notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. Neuron 2012; 74:65-78; PMID:22500631; http://dx.doi.org/ 10.1016/j.neuron.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dray N, Bedu S, Vuillemin N, Alunni A, Coolen M, Krecsmarik M, Supatto W, Beaurepaire E, Bally-Cuif L.. Large-scale live imaging of adult neural stem cells in their endogenous niche. Development 2015; 142(20):3592-600; PMID:26395477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Edelmann K, Glashauser L, Sprungala S, Hesl B, Fritschle M, Ninkovic J, Godinho L, Chapouton P.. Increased radial glia quiescence, decreased reactivation upon injury and unaltered neuroblast behaviour underlie decreased neurogenesis in the aging zebrafish telencephalon. J Comparative Neurol accepted on 05.03.2013; 2013; 521(13):3099-115; PMID:23787922 [DOI] [PubMed] [Google Scholar]
- [21].Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G.. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011; 8:566-79; PMID:21549330; http://dx.doi.org/ 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH.. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4:1313-7; PMID:9809557; http://dx.doi.org/ 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- [23].Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J.. Neurogenesis in the Striatum of the Adult Human Brain. Cell 2014; 156:1072-83; PMID:24561062; http://dx.doi.org/ 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- [24].Ganz J, Kaslin J, Hochmann S, Freudenreich D, Brand M.. Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia 2010; 58:1345-63; PMID:20607866 [DOI] [PubMed] [Google Scholar]
- [25].Ganz J, Kroehne V, Freudenreich D, Machate A, Geffarth M, Braasch I, Kaslin J, Brand M.. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. Version 2.[v1; ref status: indexed], 2014; 3:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, et al.. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 2014; 159:775-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Götz M, Huttner WB.. The cell biology of neurogenesis. Nat Rev Mol Cell Biol 2005; 6:777-88; PMID:16314867; http://dx.doi.org/ 10.1038/nrm1739 [DOI] [PubMed] [Google Scholar]
- [28].Götz M, Sirko S, Beckers J, Irmler M.. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia 2015; 63:1452-68; PMID:25965557; http://dx.doi.org/ 10.1002/glia.22850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M.. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol 2006; 295:263-77; PMID:16682018; http://dx.doi.org/ 10.1016/j.ydbio.2006.03.040 [DOI] [PubMed] [Google Scholar]
- [30].Hartfuss E, Galli R, Heins N, Gotz M.. Characterization of CNS precursor subtypes and radial glia. Dev Biol 2001; 229:15-30; PMID:11133151; http://dx.doi.org/ 10.1006/dbio.2000.9962 [DOI] [PubMed] [Google Scholar]
- [31].Haubensak W, Attardo A, Denk W, Huttner WB.. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A 2004; 101:3196-201; PMID:14963232; http://dx.doi.org/ 10.1073/pnas.0308600100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE.. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010; 68:668-81; PMID:21092857; http://dx.doi.org/ 10.1016/j.neuron.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kempermann G. Activity Dependency and Aging in the Regulation of Adult Neurogenesis. Cold Spring Harbor Perspectives Biol 2015; 7:a018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kishimoto N, Alfaro-Cervello C, Shimizu K, Asakawa K, Urasaki A, Nonaka S, Kawakami K, Garcia-Verdugo JM, Sawamoto K.. Migration of neuronal precursors from the telencephalic ventricular zone into the olfactory bulb in adult zebrafish. J Comparative Neurol 2011; 519:3549-65; PMID:21800305; http://dx.doi.org/ 10.1002/cne.22722 [DOI] [PubMed] [Google Scholar]
- [35].Kizil C, Kyritsis N, Dudczig S, Kroehne V, Freudenreich D, Kaslin J, Brand M.. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Developmental Cell 2012; 23:1230-7; PMID:23168169; http://dx.doi.org/ 10.1016/j.devcel.2012.10.014 [DOI] [PubMed] [Google Scholar]
- [36].Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M.. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 2011; 138:4831-41; PMID:22007133; http://dx.doi.org/ 10.1242/dev.072587 [DOI] [PubMed] [Google Scholar]
- [37].Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M.. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012; 338:1353-6; PMID:23138980; http://dx.doi.org/ 10.1126/science.1228773 [DOI] [PubMed] [Google Scholar]
- [38].Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. . Dynamic Contribution of Nestin-Expressing Stem Cells to Adult Neurogenesis. J Neurosci 2007; 27:12623-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lois C, Alvarez-Buylla A.. Long-distance neuronal migration in the adult mammalian brain. Science 1994; 264:1145-8; PMID:8178174; http://dx.doi.org/ 10.1126/science.8178174 [DOI] [PubMed] [Google Scholar]
- [40].Lugert S, Vogt M, Tchorz JS, Muller M, Giachino C, Taylor V.. Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat Commun 2012; 3:670; PMID:22334073; http://dx.doi.org/ 10.1038/ncomms1670 [DOI] [PubMed] [Google Scholar]
- [41].Lyons DA, Guy AT, Clarke JDW.. Monitoring neural progenitor fate through multiple rounds of division in an intact vertebrate brain. Development 2003; 130:3427-36; PMID:12810590; http://dx.doi.org/ 10.1242/dev.00569 [DOI] [PubMed] [Google Scholar]
- [42].März M, Chapouton P, Diotel N, Vaillant C, Hesl B, Takamiya M, Lam CS, Kah O, Bally-Cuif L, Strähle U.. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 2010; 58:870-88. [DOI] [PubMed] [Google Scholar]
- [43].März M, Schmidt R, Rastegar S, Strähle U.. Regenerative response following stab injury in the adult zebrafish telencephalon. Developmental Dynamics 2011; 240:2221-31; http://dx.doi.org/ 10.1002/dvdy.22710 [DOI] [PubMed] [Google Scholar]
- [44].Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M.. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 2004; 131:3133-45; PMID:15175243; http://dx.doi.org/ 10.1242/dev.01173 [DOI] [PubMed] [Google Scholar]
- [45].Ninkovic J, Mori T, Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci 2007; 27:10906-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR.. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004; 7:136-44; PMID:14703572; http://dx.doi.org/ 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- [47].Obernier K, Tong CK, Alvarez-Buylla A.. Restricted nature of adult neural stem cells: re-evaluation of their potential for brain repair. Frontiers Neurosci 2014; 8:162; PMID:24987325; http://dx.doi.org/ 10.3389/fnins.2014.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ortega FG, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B.. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol 2013; 15:602-13; PMID:23644466; http://dx.doi.org/ 10.1038/ncb2736 [DOI] [PubMed] [Google Scholar]
- [49].Pilz GA, Shitamukai A, Reillo I, Pacary E, Schwausch J, Stahl R, Ninkovic J, Snippert HJ, Clevers H, Godinho L, et al.. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat Commun 2013; 4:2125; PMID:23839311; http://dx.doi.org/ 10.1038/ncomms3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A.. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci 2013; 110:E1045-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Portavella M, Torres B, Salas C.. Avoidance Response in Goldfish: Emotional and Temporal Involvement of Medial and Lateral Telencephalic Pallium. J Neurosci 2004; 24:2335-42; PMID:14999085; http://dx.doi.org/ 10.1523/JNEUROSCI.4930-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, et al.. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun 2013; 4:2049; PMID:23804023; http://dx.doi.org/ 10.1038/ncomms3049 [DOI] [PubMed] [Google Scholar]
- [53].Rothenaigner I, Krecsmarik M, Hayes JA, Bahn B, Lepier A, Fortin G, Gotz M, Jagasia R, Bally-Cuif L.. Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development 2011; 138:1459-69; PMID:21367818; http://dx.doi.org/ 10.1242/dev.058156 [DOI] [PubMed] [Google Scholar]
- [54].Seri B, García-Verdugo JM, McEwen BS, Alvarez Buylla A.. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neuroscience 2001; 21:7153-60; PMID:11549726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shao J, Chen D, Ye Q, Cui J, Li Y, Li L.. Tissue regeneration after injury in adult zebrafish: The regenerative potential of the caudal fin. Developmental Dynamics 2011; 240:1271-7; PMID:21412938; http://dx.doi.org/ 10.1002/dvdy.22603 [DOI] [PubMed] [Google Scholar]
- [56].Shook BA, Manz DH, Peters JJ, Kang S, Conover JC.. Spatiotemporal changes to the subventricular zone stem cell pool through aging. J Neurosci 2012; 32:6947-56; PMID:22593063; http://dx.doi.org/ 10.1523/JNEUROSCI.5987-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sierra A, Martín-Suárez S, Valcárcel-Martín R, Pascual-Brazo J, Aelvoet SA, Abiega O, Deudero JJ, Brewster AL, Bernales I, Anderson AE, et al.. Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell 2015; 16:488-503; PMID:25957904; http://dx.doi.org/ 10.1016/j.stem.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Skaggs K, Goldman D, Parent JM.. Excitotoxic brain injury in adult zebrafish stimulates neurogenesis and long-distance neuronal integration. Glia 2014; 62(12):2061-79, n/a-n/a; PMID:25043622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, et al.. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013; 153:1219-27; PMID:23746839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, et al.. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2002; 22:299-307; PMID:11891435; http://dx.doi.org/ 10.1097/00004647-200203000-00007 [DOI] [PubMed] [Google Scholar]
- [61].Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O.. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 2006; 24:739-47; PMID:16210404; http://dx.doi.org/ 10.1634/stemcells.2005-0281 [DOI] [PubMed] [Google Scholar]
- [62].Tonchev AB, Yamashima T, Sawamoto K, Okano H.. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. J Neurosci Res 2005; 81:776-88; PMID:16047371; http://dx.doi.org/ 10.1002/jnr.20604 [DOI] [PubMed] [Google Scholar]
- [63].Tong CK, Fuentealba LC, Shah JK, Lindquist RA, Ihrie RA, Guinto CD, Rodas-Rodriguez JL, Alvarez-Buylla A.. A Dorsal SHH-dependent domain in the V-SVZ produces large numbers of oligodendroglial lineage cells in the postnatal brain. Stem Cell Reports 2015; 5:461-70; PMID:26411905; http://dx.doi.org/ 10.1016/j.stemcr.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Vargas JP, Rodríguez F, López JC, Arias JL, Salas C.. Spatial learning-induced increase in the argyrophilic nucleolar organizer region of dorsolateral telencephalic neurons in goldfish. Brain Res 2000; 865:77-84; PMID:10814734; http://dx.doi.org/ 10.1016/S0006-8993(00)02220-4 [DOI] [PubMed] [Google Scholar]
- [65].Zupanc GKH, Ott R.. Cell proliferation after lesions in the cerebellum of adult teleost fish: time course, origin, and type of new cells produced. Exp Neurol 1999; 160:78-87; PMID:10630192; http://dx.doi.org/ 10.1006/exnr.1999.7182 [DOI] [PubMed] [Google Scholar]


