Figure 10.
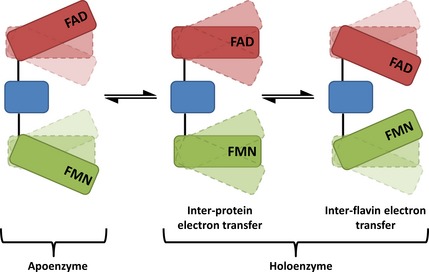
Schematic representation of the conformational equilibria of CPR. The NADP/FAD‐binding domain is shown in red, the FMN‐binding domain is shown in green, and the connecting domain is shown in blue. The NADP +‐free form of CPR adopts predominantly more open conformations, with relatively larger distances between the flavin cofactors. Upon coenzyme addition (NADP +/NADPH), oxidized CPR makes a transition to more compact forms, with relatively shorter inter‐flavin distances. Transfer of a hydride anion from NADPH to FAD causes a shift in the conformational sub‐states to predominantly more compact CPR conformations. These compact forms favour electron transfer from FADH 2 to FMN (i.e. short electron transfer distances relative to more open conformations). When CPR is reduced with excess NADPH, an additional opening phase is observed after initial closing triggered by (stoichiometric) NADPH reduction. This opening allows the enzyme to transfer electrons rapidly from the FMN domain of CPR to partner proteins. Electron transfer between cofactors and partner proteins may occur in multiple conformational states. However, the model implies that these reactions will be more rapid in selected conformational sub‐states in which donor–acceptor distances are shortened.
