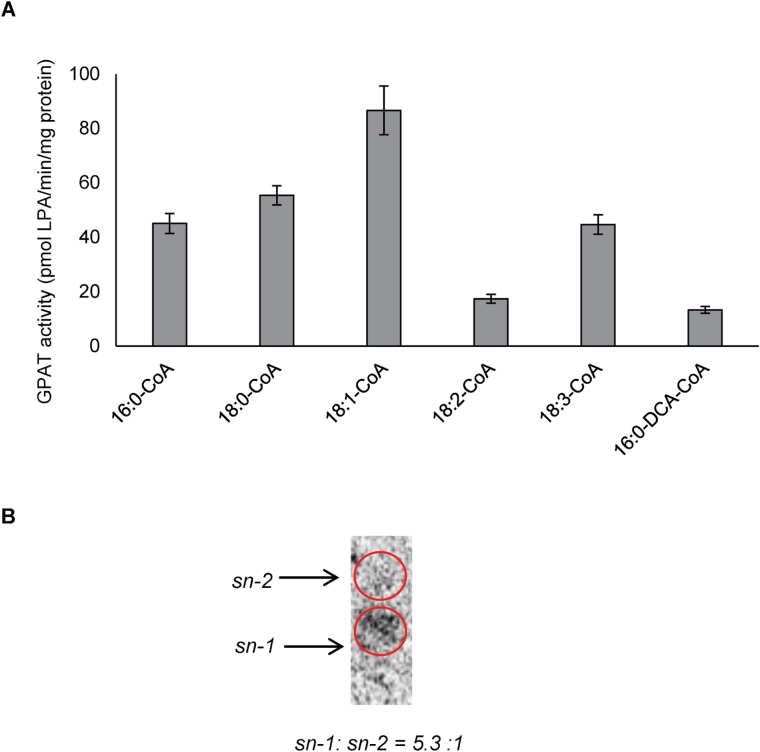
Fig. 1.
Substrate specificity and regio-specificity of Arabidopsis GPAT9. (A) In vitro enzyme assays of GPAT9 indicate that it prefers acyl-CoA over dicarboxylic acid (DCA)-CoA as a substrate. Bars represent mean enzyme activities when the enzyme was assayed at 22 ºC for 20min. Standard errors of three independent enzyme preparations are also shown. Microsomes were prepared from yeast cells. Saccharomyces cerevisiae strain gat1Δ (Matα, his3C1, leu2C0, lys2C0, ura3C0, YKR067w::kanMX4; Zheng and Zou, 2001) was transformed with Arabidopsis GPAT9 and an empty vector control, respectively. (B) In vitro regio-specificity assays of GPAT9 reveal the enzyme is an sn-1 acyltransferase. Regio-specificity was determined by de-phosphorylating the lysophosphatidic acid (LPA) product, generated in the enzyme assays, using a commercial E. coli alkaline phosphatase and subsequently separating the resulting sn-1 and sn-2 monoacylglycerol on a borate-TLC plate.