Figure 2.
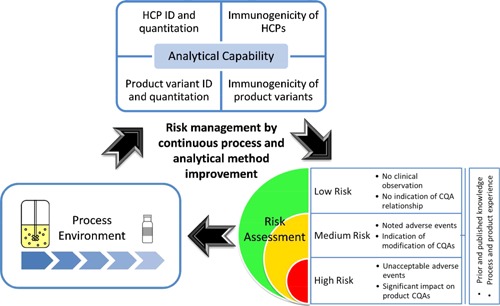
The risk assessment continuum is presented in the context of biopharmaceutical process development. In the risk assessment phase, risk is identified and assessed using prior and existing knowledge to identify which parameters may impact the critical quality attributes (CQA). Then a risk mitigation or reduction action is taken. This could be acceptance of the risk or process modification to ensure risk reduction. Then the risk assessment is repeated to determine if acceptable or not. As indicated, this is all mediated by the organization's analytical capability.
