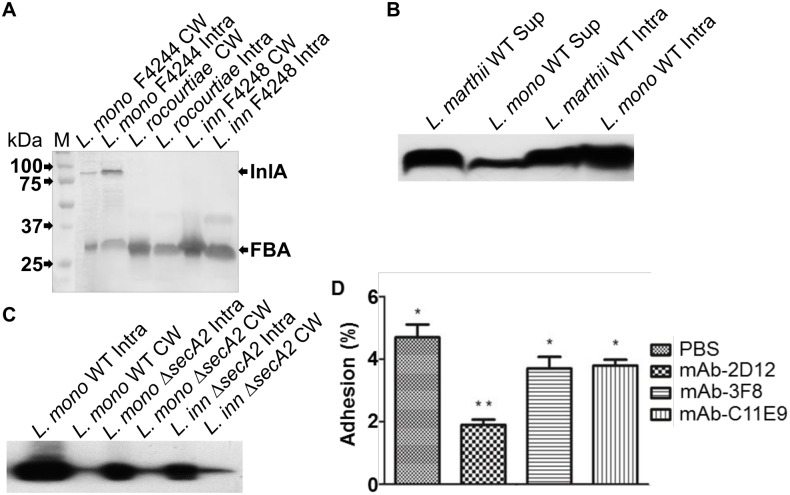
Fig 3. Cell localization and adhesion experiments for FBA characterization.
(A) Western blot using mAb-3F8 show that FBA protein is present in the cell wall and intracellular (membrane and cytoplasm) of three different Listeria species tested. The top band shows reaction with anti-InlA mAb-2D12. (B) When using only mAb-3F8 in Western blot, it is possible to observe that FBA is also present in the culture supernatant of two different Listeria species. (C) Due to the presence of FBA in the cell wall, a L. monocytogenes mutant (ΔsecA2) was used to inquire the secretion pathway of FBA. However, no difference in band intensity was observed in the cell wall (CW) or intracellular (Intra) fractions between the wild type (WT) and mutant strains, indicating that the secretory pathway of FBA is not SecA2-dependent. (D) Adhesion experiments in vitro using HCT-8 cells show that mAb-3F8 has no inhibition activity, since it’s pre-incubation with L. monocytogenes cells shows similar levels of adhered bacteria as the negative controls with PBS and mAb-C11E9. The positive control mAb-2D12 (anti-InlA) was the only one to show significant reduction in adhesion.