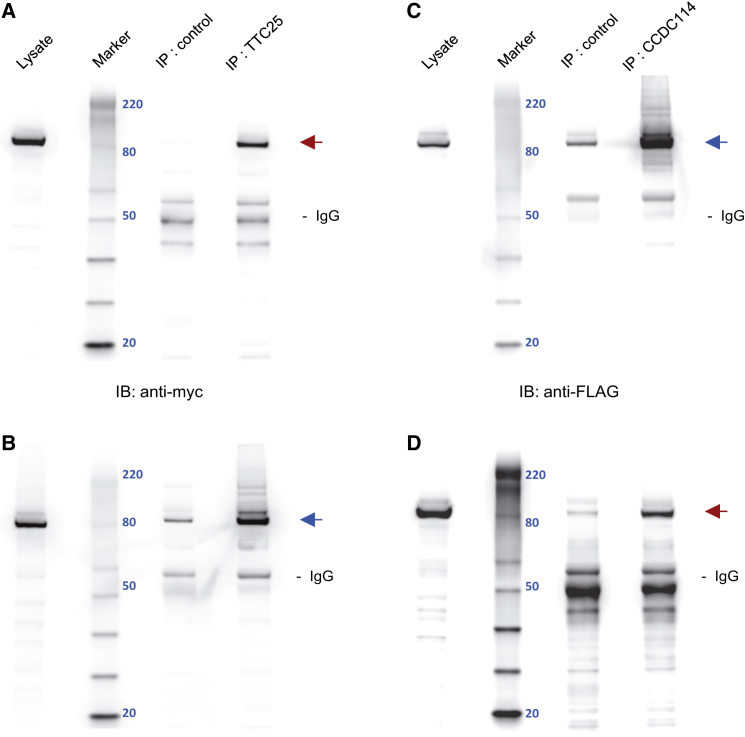
Figure 6.
TTC25 Reciprocally Co-immunoprecipitates with the ODA-DC Protein CCDC114
Following co-expression of myc-tagged TTC25 and FLAG-tagged CCDC114 in HEK293 cells, lysates were immunoprecipitated with rabbit control and either rabbit anti-TTC25 or rabbit anti-CCDC114 antibodies.
(A and B) Rabbit anti-TTC25 immunoprecipitates myc-tagged TTC25 (red arrow) and FLAG-tagged CCDC114 (blue arrow), as detected by immunoblotting (IB) with mouse anti-myc (Abcam, ab18185, 1:2000) and anti-FLAG (Sigma, F1804, 1:2000) antibodies.
(C and D) Rabbit anti-CCDC114 immunoprecipitates FLAG-tagged CCDC114 (blue arrow) and myc-tagged TTC25 (red arrow), as detected by immunoblotting with mouse anti-myc and anti-FLAG antibodies. Relative molecular weight in kDa is visualized with a Magic Mark XP protein ladder and indicated by blue numerals (Marker). Lysate equals approximately 25 μg of protein. Immunoprecipitate fractions represent 1/16 lysis volume (33 μl); 16 μl of immunoprecipitate were electrophoresed. Immunoreactive bands in the range of 50 kDa most likely indicate HC IgG. The estimated molecular weights of TTC25 (77 kDa) and CCDC114 (75 kDa) are slightly higher due to additional peptide sequence from myc and FLAG epitopes, respectively. Residual immunoreactivity in control lanes most likely represents non-specific binding of overexpressed proteins to protein agarose A beads. Normal rabbit IgG (Santa Cruz Biotechnology, sc-2027) was used as control for immunoprecipitations.