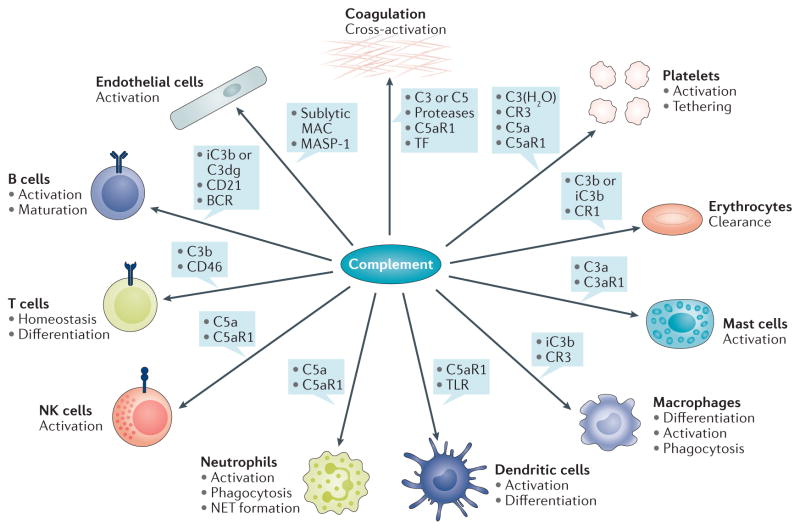
Figure 2. Examples of complement crosstalk with immune cells and defence pathways.
The generation of complement effectors stimulates a broad spectrum of downstream immune, inflammatory, and procoagulative responses. The anaphylatoxin C5a, for example, exerts strong proinflammatory effects by acting as a chemoattractant and stimulator of various immune cells via C5aR1-mediated signalling, thereby influencing priming and activation with release of mediators (for example, cytokines, neutrophil extracellular traps (NETs)), differentiation, and functional activity. C3a has a distinct spectrum from C5a and has, for example, been shown to activate mast cells. Activation of professional phagocytes induces the expression of complement receptors, which enable complement-mediated phagocytosis, whereas crosstalk between C5aR, FcγR, and dectin-1 also affects antibody-mediated uptake. Adherence of opsonins to CR1 on erythrocytes is an important mechanism that directs immune complexes to the liver and spleen. Complement activation also modulates adaptive immune responses by lowering the threshold of B-cell stimulation (via iC3b or C3dg interaction with CD21) or by influencing T-cell activation (for example, by binding of C3b to CD46), differentiation, and homeostasis. Complement effectors such as C5a, sublytic membrane attack complex (MAC), and MASP-1, can directly activate endothelial cells and, for example, increase expression of tissue factor (TF) as an inducer of coagulation. Serine proteases of the complement and coagulation systems might cross-activate under certain circumstances to contribute to thrombo-inflammation. Concomitantly, the release of complement proteins and binding of both complement activators and regulators to platelets might amplify the platelet response and contribute to clearance of platelets and pathogens alike. BCR, B-cell receptor; NK, natural killer; TLR, Toll-like receptor.