Abstract
Cadmium resistant bacterium, isolated from industrial wastewater, was characterized as Salmonella enterica 43C on the basis of biochemical and 16S rRNA ribotyping. It is first ever reported S. enterica 43C bared extreme resistance against heavy metal consortia in order of Pb2+>Cd2+>As3+>Zn2+>Cr6+>Cu2+>Hg2+. Cd2+ stress altered growth pattern of the bacterium in time dependent manner. It could remove nearly 57 % Cd2+ from the medium over a period of 8 days. Kinetic and thermodynamic studies based on various adsorption isotherm models (Langmuir and Freundlich) depicted the Cd2+ biosorption as spontaneous, feasible and endothermic in nature. Interestingly, the bacterium followed pseudo first order kinetics, making it a good biosorbent for heavy metal ions. The S. enterica 43C Cd2+ processivity was significantly influenced by temperature, pH, initial Cd2+ concentration, biomass dosage and co-metal ions. FTIR analysis of the bacterium revealed the active participation of amide and carbonyl moieties in Cd2+ adsorption confirmed by EDX analysis. Electron micrographs beckoned further surface adsorption and increased bacterial size due to intracellular Cd2+ accumulation. An overwhelming increase in glutathione and other non-protein thiols levels played a significant role in thriving oxidative stress generated by metal cations. Presence of metallothionein clearly depicted the role of such proteins in bacterial metal resistance mechanism. The present study results clearly declare S. enterica 43C a suitable candidate for green chemistry to bioremediate environmental Cd2+.
Electronic supplementary material
The online version of this article (doi:10.1186/s13568-016-0225-9) contains supplementary material, which is available to authorized users.
Keywords: Cd-resistance, S. enterica 43C, Biosorption, Glutathione, Metallothionein, smtAB gene
Introduction
Heavy metal pollution has become a major environmental problem worldwide (Ali et al. 2013). Some heavy metals including arsenic (As), cadmium (Cd), mercury (Hg) and chromium (Cr) are called non-essential metals as these are not needed for the survival of organisms and are toxic in low concentrations. Cd is highly toxic, non-essential and non-biodegradable heavy metal with half-life of 20 years (Martelli et al. 2006; Aksoy et al. 2014; Xu et al. 2014; Vinodini et al. 2015). It is soft, silver-white, electropositive metal having atomic number 48, atomic mass 112.41, density 8.64 g/cm3 and melting point 321 °C. It is divalent in all of its stable compounds and found abundantly in nature in the form of CdS. It may also combine with ammonia and cyanide to form Cd(NH3)−46 and Cd(CN)−24, respectively. Various industrial processes such as mining, electroplating, stabilizing plastics, manufacturing batteries, alloy, pigment, cement, fossil fuel combustion, municipal and sewage sludge incineration and high phosphate fertilizers are responsible for the release of huge amount of Cd2+ in the environment (Klaassen et al. 2009; Yazdankhah et al. 2010; Liu et al. 2013). According to an estimation 30,000 tons Cd2+ is released in our environment annually (Nriagu and Pacyna 1988).
Cd and almost all of its compounds are water soluble and hence easily gain entry in human food chain. No physiological role of cadmium in human cellular metabolism has been reported so far and it is extremely toxic in very minute quantity (Aksoy et al. 2014; Xu et al. 2014). It has also been reported to cause osteoporosis and fractures, anemia, eosinophilia, anosmia, apoptosis, diabetes mellitus, oncogenes activation, Itai–Itai disease and chronic pulmonary problems (Waisberg et al. 2003; Edwards and Prozialeck 2009; Yazdankhah et al. 2010).
No treatment for cadmium toxicity has been approved so far (Zhai et al. 2015). Several chemical and physical methods are used to remove cadmium from industrial effluent prior to release the effluent into the environment but all these methods are expensive and less effective (Kurniawan et al. 2006). So the use of living and dead microbial biomass is gaining attraction to remove heavy metals from environment due to less expensive, effective, time efficient and environmental friendly strategy (Feng and Aldrich 2004; He et al. 2011; Huang et al. 2014). Bacteria remove heavy metal ions including Cd2+ from the environment either by metabolism independent adsorption on their cell walls or metabolism dependent intracellular accumulation (Gadd 1990; Srinath et al. 2002; Vargas-García et al. 2012). Hyperaccumulation of Cd2+ has been reported to disturb the cell physiology by reactive oxygen species (ROS) production and disruption of bacterial respiratory proteins (Gibbons et al. 2011; Zeng et al. 2012). Bacteria have evolved several resistance mechanisms including efflux transport, precipitation, transformation and intracellular sequestration by metallothionein, glutathione and other thiol containing compounds to combat the negative effects of heavy metal ions intracellular accumulation (Nies 2003; Intorne et al. 2012; Maynaud et al. 2014).
The present study is aimed at the isolation and characterization of Cd2+ resistant bacterium from industrial wastewater and its potential use to remove Cd2+ from aqueous environment. The effect of culture conditions with reference to pH, temperature, initial Cd2+ concentration, biomass dosage and co-metal ions on the Cd2+ processing capability of the bacterium was also measured. Adsorption data was evaluated by applying adsorption isotherm models, thermodynamic and kinetic studies. Adsorption and intracellular accumulation of Cd2+ were confirmed by FTIR, SEM and EDX analysis. The effect of Cd2+ stress on bacterial growth and cell physiology (change in glutathione and non-protein thiol levels) was also determined.
Materials and methods
Sample collection
Industrial wastewater samples were collected in sterilized screw capped bottles from different industrial areas of Sheikhupura and Kotlakhpat, Lahore, Pakistan. Some physiochemical parameters of wastewater samples viz., temperature (°C), pH and cadmium ion concentration (mM) were measured.
Isolation and purification of cadmium resistant bacteria
To isolate cadmium resistant bacteria, 100 µL of wastewater sample was spread on Luria-Bertani (LB) agar (1 % NaCl, 1 % tryptone, 0.5 % yeast extract and 1.5 % agar) plates supplemented with 1 mM Cd2+. Bacterial growth was observed after incubation at 37 °C for 24 h. Single colony was picked with sterilized wire loop and re-streaked on Cd2+ supplemented LB agar plates and again incubated at 37 °C for 24 h. The process was repeated until the purified single colony obtained.
Determination of minimum inhibitory concentration
The bacterial isolate was grown in minimal salt (MS) broth (1 % (NH4)2SO4, 0.086 % CaCl2, 0.15 % K2HPO4, 0.1 % KH2PO4, 0.1 % MgSO4, 0.02 % FeSO4 and 1 % glucose) supplemented with 1 mM Cd2+ at 37 °C for 24 h. Growth was observed by taking absorbance at 600 nm. The process was repeated with high concentrations of Cd2+ until the growth of the isolate was inhibited. The minimum Cd2+ concentration at which bacterial isolate did not show growth was considered as its MIC.
Determination of optimum growth conditions
Optimum growth conditions of bacterial isolate were determined with respect to temperature and pH. To determine optimum temperature, isolate was grown in LB broth at different incubating temperatures viz., 20, 25, 30, 37 and 42 °C. After 24 h incubation, their absorbance was measured at 600 nm using spectrophotometer. To determine optimum pH, 250 mL flasks having 100 mL LB broth were prepared in six sets, each set containing three flasks, their pH were adjusted at 5, 6, 7, 8, 9 and 10 and autoclaved. These flasks were inoculated with 1 % freshly prepared culture of the isolate. After 24 h incubation at optimum temperature, absorbance was taken at 600 nm.
Effect of cadmium on bacterial growth
Effect of Cd2+ on the growth of bacterial strain was determined by growing in the presence (1 mM Cd2+) as well in the absence (control) of Cd2+. MS broth (100 mL) was taken in a set of three 250 mL flasks, autoclaved, supplemented with 1 mM Cd2+, inoculated with 1 % of freshly prepared inoculum and incubated at 37 °C in shaking incubator at 100 rpm. In one flask no Cd2+ was added which worked as a positive control. Growth in each culture was determined every 4 h by taking absorbance of an aliquot (1 mL) at 600 nm. Growth curves were plotted between time and absorbance.
Resistance to heavy metal ions
Resistance of bacterial isolate against heavy metal ions (zinc, lead, copper, chromium, arsenic and mercury) was determined by growing it in MS broth supplemented with respective metal ions. Stock solutions of 1 M concentration of heavy metal ions salts (zinc sulfate, lead nitrate, copper sulfate, potassium dichromate, sodium arsenate and mercuric chloride) were used. The concentration of metal ions was increased, 1 mM every time, in a stepwise manner until the growth of the isolate was inhibited. The bacterial growth was determined by taking optical density (OD) at 600 nm after 24 h of incubation at 37 °C.
Physical, biochemical and molecular characterization
Bacterial isolate was identified on the basis of colony morphology and different biochemical tests such as Gram staining, catalase test, oxidase test, citrate utilization, fermentation of carbohydrates, H2S production, nitrate reduction, indole and urease test (Benson 1994). For molecular characterization, 16S rRNA gene was amplified through polymerase chain reaction (PCR) by using general primers (RS-1; 5′-AAACTCAAATGAATTGACGG-3′, RS-3; 5′-ACGGGCGGTGTGTAC-3′) (Rehman et al. 2007). Amplification of 16S rRNA gene was carried out by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min and extension at 72 °C for 2 min followed by final extension at 72 °C for 5 min. The PCR product (approximately 0.5 kb) was purified through Fermentas GeneJet Gel Extraction kit (#K0691) and sequenced from Macrogen, Korea. The sequence obtained was compared with known sequences in NCBI database using NCBI BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST) to identify the bacterial isolate. The accession number was obtained by submitting the sequence in the GenBank.
Cadmium processing ability of bacterial isolate
To ascertain cadmium processing ability, the bacterial isolate was grown in 100 mL LB broth in 250 mL flask on shaking (100 rpm) at optimum temperature and pH. After 24 h of incubation the medium was supplemented with 1 mM Cd2+. In one flask Cd2+ was added in the same concentration (1 mM) but not inoculated with the organism, which worked as a control. The experiment was carried out in triplicate. Then 5 mL samples were taken out from each flask after 2, 4, 6 and 8 days. Bacterial cells were harvested through centrifugation at 3000 rpm for 5 min. The pellets [acid digested, 0.2 N HNO3 (1:1)] and supernatants were used for the estimation of Cd2+ by atomic absorption spectrophotometer at a wavelength of 228.8 nm. Each pellet was weighed and divided into two portions, one portion was washed thrice with 0.5 M EDTA to collect Cd2+ adsorbed on the cell surface and second portion was acid digested to release absorbed Cd2+. Total uptake values were calculated by subtracting the metal concentration in treated flasks from the metal concentration in control flask. Standard curve was prepared to calculate amount of metal ions in pellets and supernatants. Percentage increase and decrease in the amount of metal ions were also calculated.
Removal of Cd2+ from industrial effluent
A lab experiment was set up to determine the efficacy of bacterial isolate to remove Cd2+ from industrial wastewater. Our set up (experiment design) consisted of three plastic containers; one container carrying 10 L distilled water along with 1.5 L bacterial culture grown to log phase, second container carrying 10 L industrial effluent along with 1.5 L bacterial culture while third container was carrying only 10 L industrial effluent. Experiment was carried out at room temperature (25 ± 2 °C) and 1 mM cadmium stress was maintained in each container. Samples (10 mL) were taken from each container after 2, 4, 6 and 8 days of incubation and cells were harvested by centrifugation at 3000 rpm for 5 min. Cell pellets were weighed and separated whereas supernatants were used to estimate the amount of Cd2+ in the wastewater and further removed by bacterial isolate.
Cd2+ removal by heat-inactivated bacterial cells
Biosorption of Cd2+ by heat inactivated bacterial cells was studied by growing the isolate in 250 mL Enlermayer flask containing 100 mL LB broth medium at 37 °C in shaking incubator (150 rpm). After 24 h of incubation bacterial cells were inactivated by autoclaving at 121 °C and 15 LB pressure for 15 min. Dead bacterial cells were harvested by centrifugation at 10,000 rpm for 10 min. Cells were washed with autoclaved deionized distilled water thrice and dried at 80 °C in oven. Dead bacterial biomass (0.5 g) was added in flask containing 100 mL of 1 mM Cd2+ solution and incubated at 37 °C on shaking (150 rpm). After regular time intervals samples were collected and centrifuged at 3000 rpm for 5 min. Pellets and supernatants were used to estimate the quantity of Cd2+ by atomic absorption spectrophotometer. The experiment was performed in triplicate.
Effect of temperature, pH, initial Cd2+ concentration, biomass dosage and co-metal ions on Cd2+ processing ability of bacterial biomass
To ascertain the effect of temperature and pH on the Cd2+ processing ability of the bacterial isolate, it was grown in LB broth to log phase in flasks on optimum conditions, then 1 mM Cd2+ stress was given in each flask and incubated at different incubating temperatures (25, 30, 37 and 42 °C) and pH (5, 6, 7, 8 and 9). The effect of initial Cd2+ concentration on the Cd2+ processing ability was checked by growing the isolate in different initial concentration of Cd2+ ranging from 0.5 to 5 mM. Presence of competing metal ions is known to have effect on the biosorption ability. Their effect was studied by growing the isolate in the presence of 1 mM Cd2+ along with 1 mM of one of the competing ions (Cr6+, Pb2+ and Cu2+). A mixture of four metal ions (Cd2+, Cr6+, Pb2+ and Cu2+) was also used to observe co-metal ions effect. To study the effect of biomass concentration, bacterial isolate was grown in the presence of different initial biomass concentration ranging from 5 to 50 g/L. A blank solution containing only Cd2+ was also run as a control in each experiment. After 6 days of incubation, samples (5 mL) were collected and centrifuged at 3000 rpm for 5 min. Pellets were separated, dried and weighed whereas supernatants were used to estimate Cd2+ by atomic absorption spectrophotometer. All the experiments were performed in triplicates.
Biosorption isotherms, thermodynamics and kinetics
The amount of Cd2+ removed by the living and dead biomass of the bacterial isolate at time t was calculated by the following equation;
where q is Cd2+ uptake (mM of Cd2+ removed/g of biomass) at time t, V is sample volume taken (mL), Ci is the Cd2+ concentration initially added in the medium (mM), Cf is the final Cd2+ concentration (mM) remained in the medium after time t and m is the weight of bacterial biomass (g).
The adsorption capacity at equilibrium (qe) was calculated by using following equation.
where Ce is the Cd2+ concentration at equilibrium.
The Langmuir and Freundlich isotherm equations were applied to Cd2+ uptake data by the bacterial isolate.
Langmuir equation is represented as
The linearized form of the equation is
Values of qmax and b were calculated by drawing a plot of Ce/qe vs Ce.
where qe refers to the amount of metal ions sorbed at equilibrium (mM/g), Ce is the metal ions concentration in solution at equilibrium (mM), qmax is the maximum sorption capacity of biosorbent (mM/g) and b is Langmuir constant.
A dimensionless constant separation factor (RL) was calculated to express the Langmuir isotherm feasibility by using following equation;
where b is Langmuir constant and Ci is the initial concentration of metal ion (Cd2+).
Freundlich isotherm model reveals the sorption of metal ions on heterogenous surfaces forming multilayers. Freundlich equation is written as
Its linearized form is written as
The values of Kf and 1/n can be calculated by drawing plot of ln qe vs ln Ce.
Standard thermodynamic parameters i.e. Gibbs free energy change (∆G°), enthalpy change (∆H°) and entropy change (∆S°) were calculated by using following equations;
where R is universal gas constant (8.314 J/mol K), T refers to temperature (K) and KD (qe/Ce) is distribution coefficient. The values of ∆S° and ∆H° were calculated by drawing plot of ln KD vs 1/T.
The dynamics of Cd2+ adsorption by bacterial cells were evaluated by applying pseudo first order and pseudo second order models. The linearized form of pseudo first order model is
where qe and qt are the adsorption capacity of Cd2+ at equilibrium and given time, t is temperature and k1 is rate constant. The value of k1 was calculated by drawing plot of ln(qe−qt) vs t.
The linearized form of pseudo second order model is;
k2 (second order rate constant) was calculated by plot of t/qt vs t.
Estimation of glutathione (GSH) and other non-protein thiol contents
Reduced and oxidized GSH and total thiol contents were estimated according to Shamim and Rehman (2015).
Protein extraction and SDS–PAGE
Bacterial total protein profiling was done according to Laemmli (1970).
Amplification of smtAB
An internal fragment of 480 bp of known smtAB gene was amplified from both plasmid and genomic DNA through PCR using known primers (smtAB-F; 5′-GAT CGACGTTGCAGA GACAG-3′, smtAB-R; 5′-GATCGAGGGCGTTTTGATAA-3′) reported by Naz et al. (2005). The reaction mixture (20 µL) contained 0.2 mM dNTPs, 20 pM each primer, 10 ng DNA, 0.25 U taq polymerase, 50 mM KCl buffer and 1.5 mM MgCl2. Amplification was carried out by 35 cycles of denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min and extension at 72 °C for 2 min followed by final extension at 72 °C for 5 min.
Fourier transform infrared spectroscopy (FTIR)
For FTIR analysis bacterial samples were prepared according to Deokar et al. (2013). Briefly, the bacterium was grown in the presence and absence of 1 mM Cd2+ for 24 h at 37 °C. The bacterial cells were harvested by centrifugation at 3000 rpm for 10 min and washed several times with saline solution (0.9 % NaCl, pH 6.5). Then bacterial pellets were freeze-dried overnight and their infrared spectra were recorded on a FTIR spectrometer (Bruker, alpha) in the region 4000–500/cm.
Scanning electron microscopy (SEM)
To prepare samples for scanning electron microscopy a drop of bacterial suspension (both untreated and treated with 1 mM Cd2+) was mounted on aluminum stub and air dried. Bacterial cells were fixed with 2.5 % glutaraldehyde in phosphate buffer saline (pH 7.2), incubated at room temperature for 30 min and washed thrice with Na-phosphate buffer. Samples were dehydrated with graded acetone series (30, 50, 70, 80, 90 and 100 %) for 10 min for each step. For better contrast, samples were coated with gold film by sputter coater (Denton, Desk V HP) operating at 40 mA for 30 s under vacuum and analyzed by scanning electron microscope (Nova NanoSEM 450) equipped with Oxford energy dispersive X-ray (EDX) microanalysis system.
Statistical analysis
Observations were made and all the experiments run in triplicate. At least three separate flasks were usually maintained for one treatment. Each time three readings were taken, their mean, and standard error of the mean were calculated.
The sequences of the 16S rRNA gene was submitted to Genbank under the accession number of KJ880038.
Results
Physiochemical characteristics of wastewater
Some physicochemical parameters of wastewater were measured at the time of sampling. Temperature of different samples ranged between 25 and 40 °C, pH was between 6.2 and 9.0 and Cd2+ ranged between 0.0042 ± 0.01 and 0.0139 ± 0.01 mM.
Screening of Cd2+ resistant bacteria
The wastewater samples were spread on LB agar plates supplemented with 1 mM Cd2+. The Cd2+ concentration was increased gradually and a bacterium which showed highest resistance against Cd2+ (13.3 mM) was selected for further studies and dubbed as 43C.
Identification of bacterial isolate
Bacterial isolate was identified on the basis of morphological, biochemical characteristics and 16S rRNA ribotyping. Morphological and biochemical characteristics of the isolate are given in Table 1. The partial sequence of 16S rRNA gene showed 99 % homology with 16S rRNA sequence of Salmonella enterica (Accession number N890524.1) already submitted to NCBI database. Interestingly its identity with S. enterica subsp. enterica serovar typhimurium (ATCC 13311) is nearly 93 %, therefore, it can be regarded as non-pathogenic. The sequence of the 16S rRNA gene was submitted to Genbank under the accession number of KJ880038. The dendrogram on the basis of homology was also created (Additional file 1: Figure S1). The organism has been deposited at First Fungal Culture Bank of Pakistan with FCBP no. 590.
Table 1.
Morphological and biochemical characteristics of S. enterica 43C
| Form | Irregular |
| Surface | Smooth |
| Color | Off white |
| Margin | Entire |
| Elevation | Flat |
| Opacity | Opaque |
| Gram staining | Ve− |
| Catalase | Ve+ |
| Oxidase | Ve− |
| Citrate | Ve− |
| Lactose fermentation | Ve− |
| H2S production | Ve− |
| Nitrate | Ve− |
| Indole | Ve− |
| Urease | Ve− |
| Voges Proskauer (VP) | Ve− |
Ve− negative, Ve+ positive
Optimum growth conditions
The most suitable temperature for S. enterica 43C was found to be 37 °C and it revealed maximum growth at pH 7. The effect of Cd2+ on the growth of S. enterica 43C was monitored by growing it in the presence (1 mM Cd2+) and absence of Cd2+. The presence of Cd2+ significantly decreased the growth of the bacterium (Fig. 1).
Fig. 1.
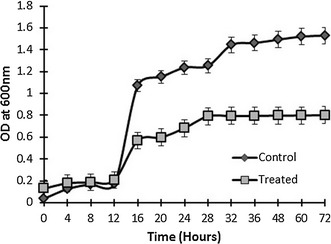
Growth pattern of S. enterica 43C in the absence (control) and presence (treated) of 1 mM Cd2+
Cross metal resistance
The bacterium resisted Cd2+ up to 13.3 mM. It was also capable to resist other heavy metal ions, viz., Zn2+ (5.3 mM), Pb2+ (16 mM), Cu2+ (2.6 mM), Cr6+ (5.3 mM), As3+ (8.8 mM) and Hg2+ (0.53 mM). The order of resistance regarding metal ions concentration was Pb2+>Cd2+>As3+>Zn2+>Cr6+>Cu2+>Hg2+.
Biosorption of Cd2+
Biosorption capability of S. enterica 43C was assessed by growing it in culture medium supplemented with 1 mM Cd2+ (Fig. 2a). Cd2+ biosorption efficiency (q) of S. enterica 43C was as 7, 11.5, 18 and 22 mM/g after 2, 4, 6 and 8 days, respectively. The amount of Cd2+ found accumulated within bacterial cells after 2, 4, 6 and 8 days was 4.5, 9, 14 and 15.7 mM/g, respectively. While 2.18, 2.2, 3.6 and 6 mM/g of Cd2+ was found to be adsorbed on the bacterial surface after 2, 4, 6 and 8 days, respectively (Fig. 2a).
Fig. 2.
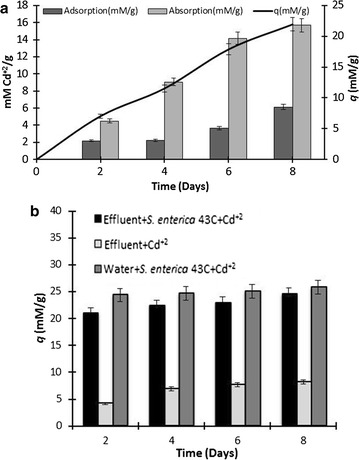
a Biosorption capability of S. enterica 43C growing in culture medium supplemented with 1 mM Cd2+ and b large scale biosorption of Cd2+ by S. enterica 43C
At large scale S. enterica 43C was able to remove 41, 43, 45.8 and 49 mM/g 83.4 % Cd2+ from industrial effluent and 43.8, 45, 50 and 51.5 mM/g from distilled water after 2, 4, 6 and 8 days, respectively. Cd2+ concentration was maintained at 1 mM in both water and industrial effluent. The microorganisms originally present in industrial effluent could remove 4, 9, 12 and 16 mM/g after 2, 4, 6 and 8 days, respectively from original industrial effluent in which no Cd2+ was added but originally contained 0.18 mM Cd2+ (Fig. 2b).
Cd2+ removal by heat-inactivated bacterial cells
Bacterial cells were inactivated by autoclaving to use as biosorbant material for Cd2+ bioremediation. Dead cells of S. enterica 43C were observed removing 8.8, 15, 21 and 23.8 mM/g Cd2+ from aqueous medium, out of which 4.27, 7.2, 10.84 and 11.06 mM/g Cd2+ was detected adsorbed on outer surface whereas 4.91, 7.92, 10.6 and 12.79 mM/g Cd2+ found accumulated within dead yeast cells after 2, 4, 6 and 8 days, respectively (Additional file 1: Figure S2).
Biosorption isotherms, thermodynamics and kinetics
Straight lines were obtained when we evaluated our data by applying Langmuir and Freundlich isotherm equations (Figs. 3, 4a–e) which suggested that our data is correlated with both the isotherm models. The values of Langmuir constants (qmax and b) and Freundlich constant (Kf and n) are given in Additional file 1: Table S1. The regression coefficient (R2) values for Langmuir isotherm ranged 0.933–0.999 whereas these values were found in the range of 0.79–0.995 for Freundlich isotherm (Additional file 1: Table S1) which is an indication that our data is more correlated with Langmuir isotherm model than Freundlich isotherm model. The values of RL (dimensionless constant separation factor which expresses the Langmuir isotherm feasibility) were calculated to determine the suitability of Langmuir model and found in the range of 0.11–0.66 (Additional file 1: Table S1).
Fig. 3.
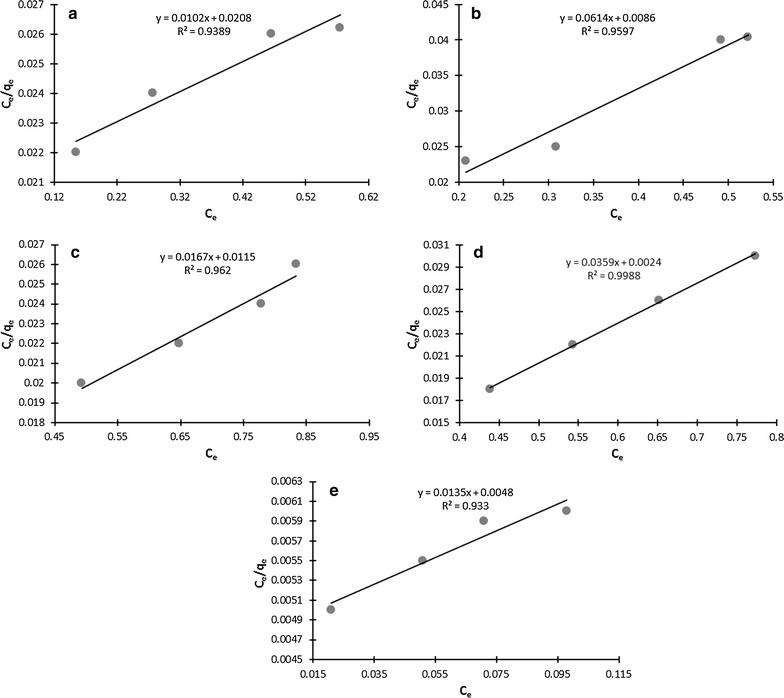
Langmuir isotherm for a living cells b dead cells c effluent plus S. enterica 43C plus Cd2+ (1 mM) d distilled water plus S. enterica 43C plus Cd2+ (1 mM) e only effluent
Fig. 4.
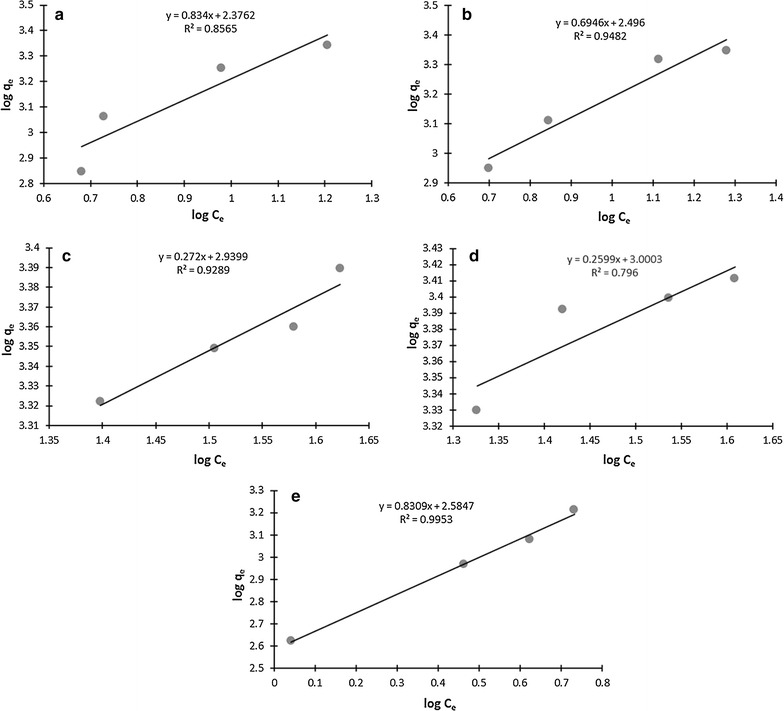
Freundlich isotherm for a living cells b dead cells c effluent plus S. enterica 43C plus Cd2+ (1 mM) d distilled water plus S. enterica 43C plus Cd2+ (1 mM) e only effluent
The standard Gibbs free energy change (∆G°) was calculated as −4.96, −5.4, −6.9, −7.5, −7.7 and −8.3 kJ/mol at 20, 25, 30, 33, 37 and 42 °C, respectively. Whereas, standard enthalpy (∆H°) and entropy (∆S°) changes were 48.9 kJ/mol and 216.4 J/mol K, respectively (Additional file 1: Table S2) from plot of ln KD vs 1/T (Additional file 1: Figure S3).
Adsorption data was evaluated by applying pseudo first and pseudo second order kinetic models. The values of constants (K1 and K2) and coefficient determinant (R2) are given in Additional file 1: Table S3. Pseudo first order R2 values are higher for lab scale experiments (0.9) than pseudo second order R2 values. Figure 5a, b showing plots of qt/t vs time(t). Furthermore, experimental q values (qexp) 21 and 9 mM/g were found in good agreement with calculated q values (qcal) 31 and 10 mM/g for living and dead cells, respectively by using pseudo first order equation. Whereas, at large scale experiments pseudo second order R2 values were higher (0.99) than pseudo first order R2 values (0.97). Moreover, qexp values and qcal values (calculated by using pseudo second order equation) were exactly similar for industrial effluent and distilled water. In both of them bacterial biomass and Cd2+ (1 mM) were added. In general, these results suggest that lab scale experiments follow pseudo first order whereas large scale experiments follow pseudo second order model. But the original effluent in which no bacterial biomass and Cd2+ were added was found following pseudo first order kinetic model just like small scale experiments.
Fig. 5.
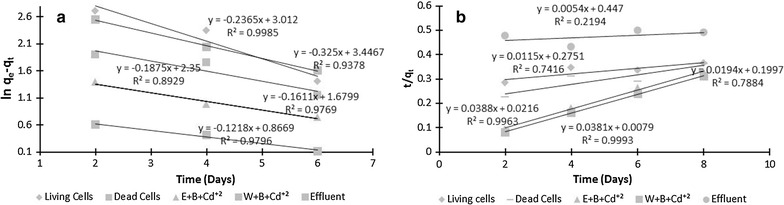
Pseudo first order (a) and pseudo second order (b) reaction for Cd2+ adsorption onto S. enterica 43C
Effect of temperature, pH, initial Cd2+ concentration, biomass dosage and co-metal ions on Cd2+ processing ability of bacterial isolate
Several parameters have been proved to have influence on the Cd2+ processing ability of S. enterica 43C. It showed optimum biosorption capability at moderate temperature (30 to 37 °C) where 15 mM/g removal of Cd2+ was observed. This removal was reduced to 0.89 mM/g when temperature was increased up to 42 °C. Similarly at low temperature (25 °C), it could remove only 13 mM/g Cd2+ from the medium (Fig. 6a). The biosorption ability of S. enterica 43C was also found changing with varying pH. Neutral (pH 7) or nearly neutral pH (pH 6) was found most suitable for S. enterica 43C and it removed 18 and 17.3 mM/g Cd2+, respectively. Basic (pH 8) and strongly basic pH (pH 9) decreased Cd2+ removal to 2.9 and 0.53 mM/g, respectively. At slightly acidic pH (pH 5), it exhibited 8.3 mM/g removal (Fig. 6b). The sorption of Cd2+ by S. enterica 43C was studied by varying initial Cd2+ concentration ranging from 0.5 to 5 mM. A direct relationship was observed between initial Cd2+ concentration and Cd2+ processing ability of S. enterica 43C 12.3, 25.8, 45, 66 and 71 mM/g sorption of Cd2+ by S. enterica 43C was recorded at 0.5, 1, 1.5, 3 and 5 mM of initial Cd2+ concentration, respectively (Fig. 6c). Figure 6d is indicating the effect of bacterial biomass dosage on the potential of S. enterica 43C to biosorp Cd2+. An increase in Cd2+ removal was evaluated with the increase in biomass concentration but up to a certain limit. Salmonella enterica 43C showed 46, 58, 66, 68 %, removal at 5, 10, 15 and 30 mg/mL of initial biomass concentrations, respectively. But biosorption efficiency (q) was decreased from 15 to 2 mM/g. Further increase (40 and 50 mg/mL) in biomass concentration did not cause any significant change in Cd2+ removal efficiency of S. enterica 43C. When S. enterica 43C was allowed to grow in the presence of Cd2+ in combinations with different heavy metal ions as competing ions, a change in bioremediation potential of S. enterica 43C was noticed. S. enterica 43C could remove 22.8, 26.6 and 12.2 mM/g Cd2+ when Cr6+, Pb2+ and Cu2+ were used as competing ions, respectively. This removal capacity was decreased to 11.7 mM/g when all these heavy metal ions i.e. Cd2+, Cr6+, Pb2+ and Cu2+ were added simultaneously. It is kept in mind that S. enterica 43C showed 21.8 mM/g removal of Cd2+ in the absence of any competing ion (Fig. 6e).
Fig. 6.
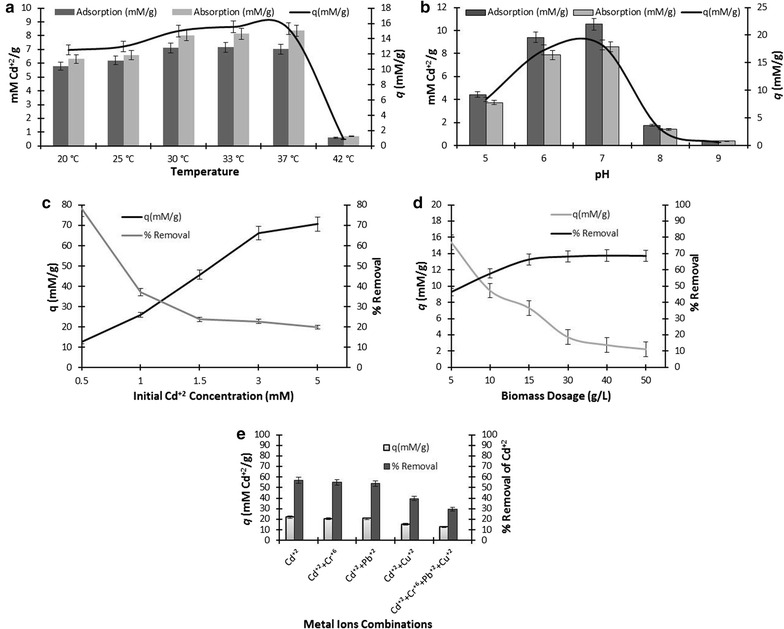
Effect of temperature (a), pH (b), initial Cd2+ concentration (c), biomass dosage (d) and co-metal ions (e) on Cd2+ processing ability of S. enterica 43C
Measurement of glutathione and non-protein thiols
The levels of GSH and GSSG were altered in the presence of Cd2+ (1 mM). There was 53.96 and 154 % increase in GSH level and GSH/GSSG ratio, respectively. Whereas 87 % increase in non-protein thiols level was recorded in bacterial cells in the presence of Cd2+ (Additional file 1: Table S4).
SDS–PAGE analysis
Total protein profiles of S. enterica 43C in the absence and presence of Cd2+ were compared by polyacrylamide gel electrophoresis. This comparison clearly indicated the induction of a protein due to Cd2+ stress. A band of about 40 kDa was observed intensifying with the increasing concentration of Cd2+ (Fig. 7a).
Fig. 7.
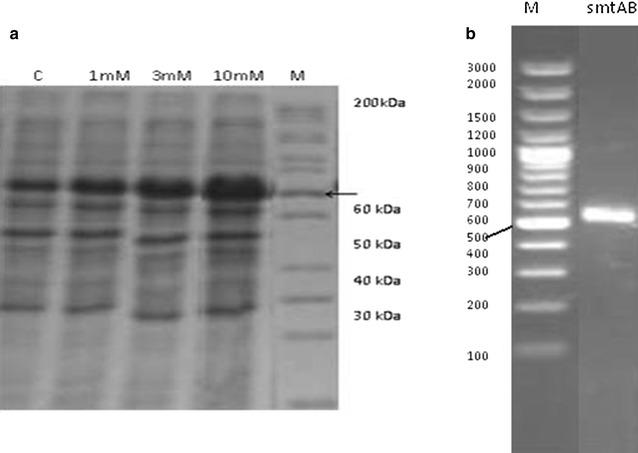
a Total protein profile of S. enterica 43C in the absence and presence of 1, 5 and 10 mM Cd2+. M protein marker; C control (without Cd2+) and b amplification of smtAB gene through PCR. M represents DNA ladder
Amplification of smtAB
PCR was carried out to amplify internal fragment of smtAB gene from both plasmid and genomic DNA of S. enterica 43C by using known primers. The primer set showed amplification of 480 bp product. But no amplification was detected when plasmid DNA was used as a template (Fig. 7b).
SEM, EDX and FTIR
The size of S. enterica 43C was observed to increase many folds, when treated with Cd2+. This indicated the accumulation of Cd2+ and it was confirmed from the difference in size, when SEM images of treated S. enterica 43C were compared with untreated bacterium (Fig. 8a). This biosorption of Cd2+ was verified by EDX (point and ID scan) analysis, while doing their SEM (Additional file 1: Figure S4). The FTIR analysis confirmed the presence of carboxyl, amino and phosphate moieties in bacterium and this analysis confirmed our assumption regarding binding of Cd2+ with bacterium. It is clear that the peaks attributed to amide linkage, appearing at 1635 and 1538/cm are shifted to 1647 and 1552/cm respectively, in the presence of Cd2+ (Fig. 8b).
Fig. 8.
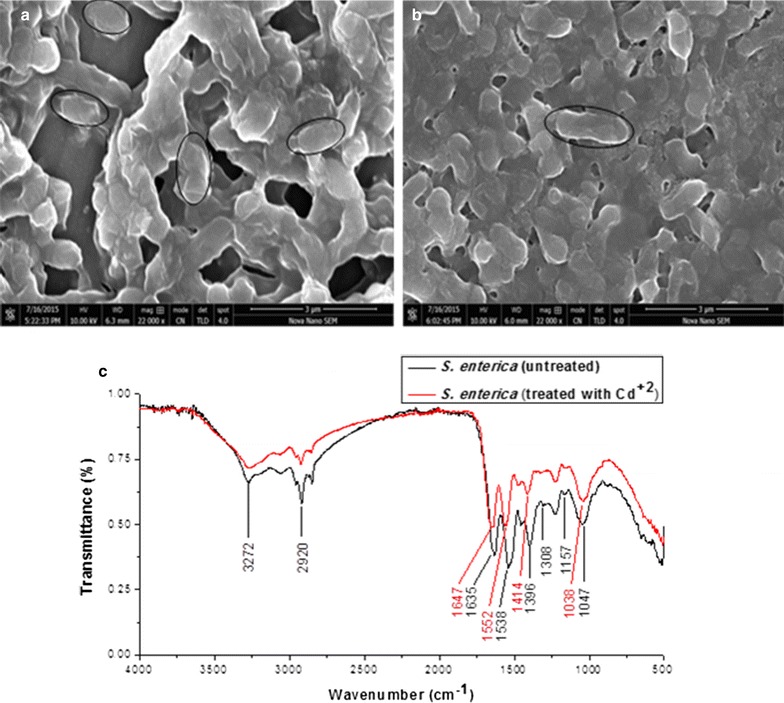
Images of scanning electron microscopy of S. enterica 43C in the absence (a) and presence (b) of Cd2+ (1 mM) and c FTIR of S. enterica 43C in the absence and presence of Cd2+
Discussion
In the present investigation we have demonstrated the potential use of S. enterica 43C to remove Cd2+ from aqueous medium at lab scale. S. enterica 43C could remove maximally 22 mM/g Cd2+ from the aqueous medium and 2.18, 2.2, 3.6 and 6 mM/g Cd2+ were found to be adsorbed on bacterial cell surface after 2, 4, 6 and 8 days, respectively. Whereas Pseudomonas stutzeri and Enterobacter sp. have been reported to remove 43.5 and 46.2 mg/g Cd2+ by adsorption (Lu et al. 2006). At large scale, S. enterica 43C maximally removed 24 mM/g (83 %) and 25 mM/g (77 %) after 8 days from effluent and water supplemented with 1 mM Cd2+. Effluent naturally contains some amount of heavy metal ions as well as number of microorganisms including bacteria, fungi, and protozoans. Experiment was also performed to check the efficacy of these naturally existing microbes to remove Cd2+. Results showed that these organisms could maximally remove only 27 % Cd2+. But our bacterium, S. enterica 43C removed 83 % Cd2+ suggesting its preferential use over other microbes for Cd2+ bioremediation.
There are several adsorption models but Langmuir and Freundlich models are commonly used (Bayramoğlu et al. 2006; Ansari and Malik 2007; Kang et al. 2007) which are based on monolayer and multilayer adsorption, respectively. In Langmuir model we make some assumptions that biosorbent surface is uniform and metal ions do not have mutual interaction and adsorb on the biosorbent surface in single layer. Additional file 1: Table S1 is showing the values of different parameters for Langmuir and Freundlich isotherm models, higher values of regression coefficient for Langmuir isotherm model indicated that our data was more consistent with Langmuir model than Freundlich model. Langmuir model is considered unfavorable for a given data if RL >1, while it is favorable when 0 < RL < 1 (Hall et al. 1966). In our experiments, RL values were found less than 1 and greater than 0 (0.11–0.66), indicating the favorability of Langmuir isotherm model. High biosorption capacity of S. enterica 43C (either living or dead form) for Cd2+ as compare to other biosorbent reported in literature (Say et al. 2001; Martins et al. 2004; Sari et al. 2008; Chatterjee et al. 2010) to bioremediate Cd2+ makes it highly potential and favorable resource to remove environmental Cd2+.
SEM revealed the increase in the size of bacterial cells in the presence of Cd2+ which clearly indicated the biosorption of Cd2+. It was further confirmed by EDX and FTIR. The characteristic peaks of carboxyl, amino and phosphate groups appearing in FTIR analysis confirmed the presence of these moieties in bacterium and the shift in these peaks was used to address the binding of Cd2+ with bacterium. In short, the peaks at 1635 and 1538/cm recognized the presence of amide linkage, and the shift in these peaks to 1647 and 1552/cm respectively was due to chemical interaction of Cd2+ with amide group of bacterium. It is presumed that cadmium mainly adsorbed on bacterium by interacting with nitrogen atoms of amide groups. The peaks appearing in range of 1000–1320/cm represented the presence of carbon and phosphorous containing oxygen atoms, and suppression in intensity of these peaks indicated their interaction with Cd2+ (Parikh and Chorover 2006). The peak at 1047 and 1157/cm recognized the presence of carbonyl group and the shift in these peaks and transmittance level was due to chemical interaction of Cd2+ with carbonyl moieties. Addition to this, the percentage transmittance of peaks in FTIR spectra of bacterium treated with cadmium, was considerably less significant than those of control bacterium. This showed that stretching of bonds, occurred to lesser degree (due to presence of Cd2+), resulted to reduce their percentage transmittance as reported by Chakravarty and Banerjee (2012a).
Biosorption is really a complex process and dependent on lot of factors such as functional groups, temperature, pH, metal ions concentration, weight of biomass and presence of competing metal ions (Lesmana et al. 2009). Among all the factors, pH and temperature have been reported as the most critical parameters in the biosorption of Cd2+ (Chakravarty and Banerjee 2012). Present investigation clearly revealed the effect of acidic, basic and neutral pH on the Cd2+ processing ability of the bacterium. Strong acidic and strong basic pH significantly decreased the biosorption of S. enterica 43C. Maximum biosorption of Cd2+ (57.14 %) has been observed at neutral pH. These results are parallel to the findings of Chakravarty and Banerjee (2012). Change in temperature also greatly influences the adsorption and overall biosorption of Cd2+. Increase in temperature not only enhances the kinetic energy of reacting molecules but also vibrational energy of constituent elements of bacterial envelop. At high temperature (above 37 °C) the vibrational energy becomes too violent resulting in the breakage of bonds between Cd2+ and functional groups on bacterial surface thus decreasing adsorption and intracellular accumulation of Cd2+ (Özdemir et al. 2009). Whereas at low temperature chances for reacting molecules to collide with each others are low, thus Cd2+ processing ability of the bacterium is declined. S. enterica 43C showed maximum Cd2+ removal (15 mM/g) at 37 °C but when the temperature was increased up to 42 °C the Cd2+ removal capability of S. enterica 43C was significantly reduced.
Initial biomass dosage showed significant effect on Cd2+ biosorption ability of S. enterica 43C. Cd2+ biosorption found increasing with the increasing biomass concentration. Highest Cd2+ biosorption (68 %) was observed when biomass dosage was increased up to 30 g/L. On contrary, biosorption efficiency (q) decreased up to 3.7 mM/g. Further increase in biomass concentration did not cause any significant effect on percentage removal and biosorption efficiency (q) of S. enterica 43C. Greater concentration of biomass provides greater surface area and binding sites for Cd2+ which increases the binding of Cd2+ with the biomass and hence increasing the percentage removal of Cd2+. However, increase in biomass concentration decreases the ratio of biosorbent to metal ions (as metal ion concentration is fixed i.e. 1 mM) thus a decline in biosorption efficiency (q) was observed (Al-Garni 2007).
Industrial wastewater contains multiple metal ions which may influence the removal of Cd2+. Present study clearly depicted the effect of other heavy metal ions on Cd2+ biosorption in binary and quaternary systems. In multimetal system the binding of metal ions to biomaterial depends on ionic properties, among them electronegativity and ionic radius are the most critical (Naja et al. 2010). Ion with larger radius has greater ability to adsorb and high electronegativity also helps the ion to bind strongly to the surface functional groups (Sulaymon et al. 2011). In the present study, Cd2+ biosorption was significantly reduced from 22 to 15 mM/g in the presence of Cu2+. Whereas Pb2+ and Cr6+ showed very slight decrease in Cd2+ biosorption in binary system. Electronegativity of Cu2+ is 1.9 while Cd2+ has 1.7 (Additional file 1: Table S2). This high electronegativity of Cu2+ makes it more preferred metal ion over Cd2+ to get adsorbed on the surface. Zhou et al. (2009) also reported this preference of Cu2+ over Cd2+.
Salmonella enterica 43C showed tolerance against Cd2+ (13.3 mM) as well as against Zn2+ (5.3 mM), Pb2+ (16 mM), Cu2+ (2.6 mM), Cr6+ (5.3 mM), As3+ (8.8 mM) and Hg2+(0.53 mM). The order of resistance regarding metal ions concentration was Pb2+>Cd2+>As3+>Zn2+>Cr6+>Cu2+>Hg2+. Salmonellaenterica 43C was found to be more tolerant against Pb2+ as compared to the Cd2+. This is due to the fact that Pb2+ is more ubiquitous heavy metal. On the other hand Cd2+ is highly toxic and its no physiological role has been reported so far (Martelli et al. 2006; Vinodini et al. 2015). Salmonella enterica 43C is notorious for its pathogenic behavior. Anyhow, pathogenicity cannot be considered as a limiting factor to use microorganisms for bioremediation purposes. Number of pathogenic bacteria including Enterobacter sp., P. aeruginosa, Shewanella and Alcaligenes faecalis have been used for bioremediation purposes (Lu et al. 2006; Srivastava and Majumder 2008; Kang et al. 2015).
Bacteria have developed different resistance mechanisms to cope with the negative effects of these metal ions (Sinha and Mukherjee 2008; Hassan et al. 2009; Khan et al. 2015) including alteration in the levels of GSH and GSSG and induction of metal binding proteins i.e. metallothioneins (Fig. 9). GSH, as a strong reducing agent, reduces the H2O2 to H2O and gets itself oxidized to GSSG (Penninckx 2002; Galano and Alvarez-Idaboy 2011). GSH/GSSG is the most important parameter to study oxidative stress. Present study clearly revealed the alteration of GSH level and GSH/GSSG. An increase of 53.96 and 154 % in GSH level and GSH/GSSG ratio was determined in the presence of Cd2+, respectively. An increase of 87 % in non-protein thiols level was also determined.
Fig. 9.
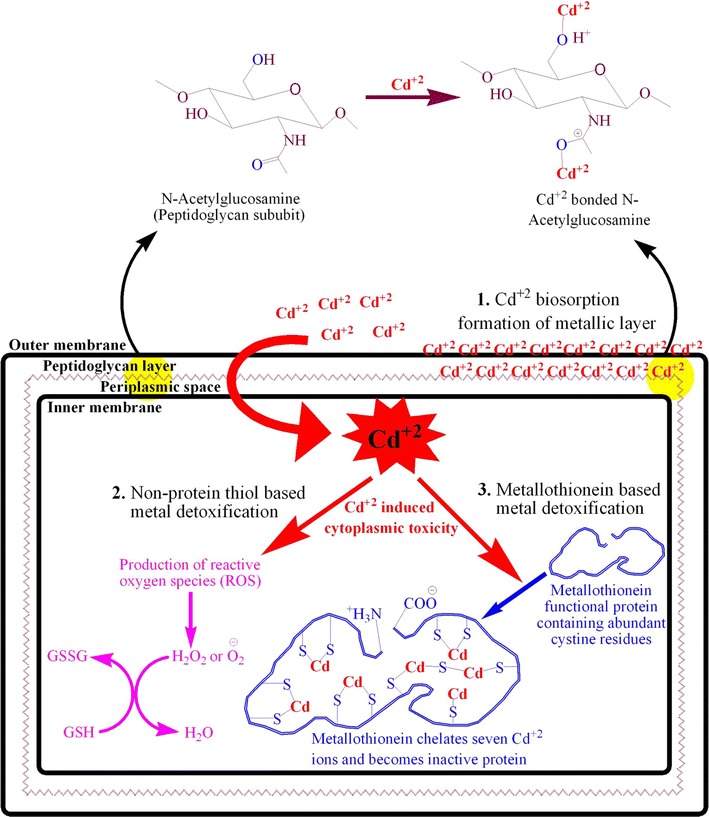
Proposed Cd2+ biosorption and resistance mechanism in gram negative bacterium, S. enterica 43C. (1) Peptidoglycan is the primary site on bacterial surface that binds Cd2+ in monolayer. (2) Cd2+ enter the cells via metal ions transport channel proteins and are known to cause oxidative stress which is combated by GSH dependent antioxidant system. (3) Metallothioneins, induced by Cd2+ stress, are primarily involved in Cd2+ sequestration through their thiols groups thus mitigating Cd2+ interference with cellular metabolism
MTs are low molecular weight (6–7 kDa), thiol-containing, cysteine rich (20–30 %), metal binding proteins, induced by cadmium and other heavy metal ions and are involved in the sequestration of metal ions (Klaassen et al. 2009; Chaturvedi et al. 2012). MTs are widely associated with eukaryotes (Palmiter 1998) and among prokaryotes, these have been reported in P. putida and Synechococcus spp. (Higham et al. 1984; Huckle et al. 1993). The amplification of smtAB gene from the chromosomal DNA of S. enterica 43C revealed the presence of genetic determinants of bacterial metallothioneins. These metallothioneins encoding genes have been found involve in conferring resistance against heavy metal ions including Cd2+ (Turner et al. 1995).
In the present study, S. enterica 43C showed high resistance against heavy metal ions in order of Pb2+>Cd2+>As3+>Zn2+>Cr6+>Cu2+>Hg2+. The bacterium could remove nearly 57 % Cd2+ from the medium over a period of 8 days. Kinetic and thermodynamic studies depicted the Cd2+ biosorption as spontaneous, feasible and endothermic in nature. The bacterium followed pseudo first order kinetics, making it a good biosorbent for heavy metal ions. The Cd2+ processing was significantly influenced by temperature, pH, initial Cd2+ concentration, biomass dosage and co-metal ions. FTIR analysis revealed the active participation of amide and carbonyl moieties in Cd2+ adsorption which was confirmed by EDX analysis. Electron micrographs beckoned further surface adsorption and increased bacterial size due to intracellular Cd2+ accumulation. An increase in GSH and other non-protein thiols levels played a significant role in thriving oxidative stress generated by metal cations. MTs presence clearly indicated the role of such proteins in bacterial metal resistance mechanism. S. enterica 43C, a non-pathogenic bacterium having strong cytoplasmic redox balance system to overcome metal induced cytotoxicity. Moreover, its pseudo first order kinetic trends make it an effective biosorbent to remove toxic heavy metals from the aqueous environment. In future, after exploring its molecular biology it can become an attractive environmental tool for green chemistry.
Authors’ contributions
ZK and AR have conceived and designed experiments. ZK, SZH, MAN, and SZ have performed the experiments. ZK, SZH and MAN have analyzed the data. AR and ARS have contributed reagents/analysis tools. ZK and AR have written the research paper. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Dr. Irshad Hussain for his advice relating to the biochemical investigations.
Competing interests
The authors declare that they have no competing interests.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This work was supported by the research Grant No. 20-1373/R&D/10 from Higher Education Commission (HEC), Islamabad, Pakistan which is gratefully acknowledged.
Abbreviations
- LB
Luria–Bertani
- PCR
polymerase chain reaction
- FTIR
fourier transform infrared
- SEM
scanning electron microscopy
- EDX
energy dispersive X-ray
- GSH
glutathione
- MTs
metallothioneins
Additional file
10.1186/s13568-016-0225-9 Tables and figures.
Contributor Information
Zaman Khan, Email: hmzamankhan@gmail.com.
Abdul Rehman, Phone: 92-42-9231249, Email: rehman_mmg@yahoo.com.
Syed Z. Hussain, Email: zajifnano@gmail.com
Muhammad A. Nisar, Email: matif100@yahoo.com
Soumble Zulfiqar, Email: soumblen@gmail.com.
Abdul R. Shakoori, Email: arshaksbs@yahoo.com
References
- Aksoy E, Salazar J, Koiwa H. Cadmium determinant 1 is a putative heavy-metal transporter in Arabidopsis thaliana. FASEB J. 2014;28(617):4. [Google Scholar]
- Al-Garni SM. Biosorption of lead by Gram-ve capsulated and non-capsulated bacteria. Water SA. 2007;31:345–350. doi: 10.4314/wsa.v31i3.5224. [DOI] [Google Scholar]
- Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals-concepts and applications. Chemosphere. 2013;91:869–881. doi: 10.1016/j.chemosphere.2013.01.075. [DOI] [PubMed] [Google Scholar]
- Ansari MI, Malik A. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour Technol. 2007;98:3149–3153. doi: 10.1016/j.biortech.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bayramoğlu G, Tuzun I, Celik G, Yilmaz M, Arica MY. Biosorption of mercury (II), cadmium (II) and lead (II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Inter J Mine Process. 2006;81:35–43. doi: 10.1016/j.minpro.2006.06.002. [DOI] [Google Scholar]
- Benson HJ. Microbiological applications. In: Wan C, editor. Laboratory manual in general microbiology. Dubuque: Brown Publishers; 1994.
- Chakravarty R, Banerjee PC. Mechanism of cadmium binding on the cell wall of an acidophilic bacterium. Bioresour Technol. 2012;108:176–183. doi: 10.1016/j.biortech.2011.12.100. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Bhattacharjee I, Chandra G. Biosorption of heavy metals from industrial waste water by Geobacillus thermodenitrificans. J Hazard Mater. 2010;175:117–125. doi: 10.1016/j.jhazmat.2009.09.136. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Mishra A, Tiwari V, Jha B. Cloning and transcript analysis of type 2 metallothionein gene (SbMT 2) from extreme halophyte Salicornia brachiata and its heterologous expression in E. coli. Gene. 2012;499:280–287. doi: 10.1016/j.gene.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Deokar AR, Lin L-Y, Chang C-C, Ling Y-C. Single-walled carbon nanotube coated antibacterial paper: preparation and mechanistic study. J Mater Chem B. 2013;1:2639–2646. doi: 10.1039/c3tb20188k. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol. 2009;238:289–293. doi: 10.1016/j.taap.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Aldrich C. Adsorption of heavy metals by biomaterials derived from the marine alga Ecklonia maxima. Hydrometallurgy. 2004;73:1–10. doi: 10.1016/S0304-386X(03)00138-5. [DOI] [Google Scholar]
- Gadd GM. Heavy metal accumulation by bacteria and other microorganisms. Experientia. 1990;46:834–840. doi: 10.1007/BF01935534. [DOI] [Google Scholar]
- Galano A, Alvarez-idaboy JR. Glutathione: mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011;1:1763–1771. doi: 10.1039/c1ra00474c. [DOI] [Google Scholar]
- Gibbons SM, Feris K, Mcguirl MA, Morales SE, Hynninen A, Ramsey PW, Gannon JE. Use of microcalorimetry to determine the costs and benefits to Pseudomonas putida strain kt2440 of harboring cadmium efflux genes. Appl Environ Microbiol. 2011;77:108–113. doi: 10.1128/AEM.01187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KR, Eagleton LC, Acrivos A, Vermeulen T. Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Indust Eng Chem Fundam. 1966;5:212–223. doi: 10.1021/i160018a011. [DOI] [Google Scholar]
- Hassan SH, Kim S-J, Jung A-Y, Joo JH, Eun OhS, Yang JE. Biosorptive capacity of Cd (II) and Cu (II) by lyophilized cells of Pseudomonas stutzeri. J Gen Appl Microbiol. 2009;55:27–34. doi: 10.2323/jgam.55.27. [DOI] [PubMed] [Google Scholar]
- He M, Li X, Liu H, Miller SJ, Wang G, Rensing C. Characterization and genomic analysis of a highly chromate resistant and reducing bacterial strain Lysinibacillus fusiformis ZC1. J Hazard Mater. 2011;185:682–688. doi: 10.1016/j.jhazmat.2010.09.072. [DOI] [PubMed] [Google Scholar]
- Higham DP, Sadler PJ, Scawen MD. Cadmium-resistant Pseudomonas putida synthesizes novel cadmium proteins. Science. 1984;225:1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- Huang F, Guo CL, Lu GN, Yi XY, Zhu LD, Dang Z. Bioaccumulation characterization of cadmium by growing Bacillus cereus RC-1 and its mechanism. Chemosphere. 2014;109:134–142. doi: 10.1016/j.chemosphere.2014.01.066. [DOI] [PubMed] [Google Scholar]
- Huckle JW, Morby AP, Turner JS, Robinson NJ. Isolation of a prokaryotic metallothionein locus and analysis of transcriptional control by trace metal ions. Mol Microbiol. 1993;7:177–187. doi: 10.1111/j.1365-2958.1993.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Intorne AC, De Oliveira MVV, De Pereira ML, De Souza Filho GA. Essential role of the czc determinant for cadmium, cobalt and zinc resistance in Gluconacetobacter diazotrophicus PAl 5. Inter Microbiol. 2012;15:69–78. doi: 10.2436/20.1501.01.160. [DOI] [PubMed] [Google Scholar]
- Kang C, Wu P, Li Y, Ruan B, Li L, Tran L, Zhu N, Dang Z. Understanding the role of clay minerals in the chromium (VI) bioremoval by Pseudomonas aeruginosa CCTCC AB93066 under growth condition: microscopic, spectroscopic and kinetic analysis. World J Microbiol Biotechnol. 2015;31:1765–1779. doi: 10.1007/s11274-015-1928-9. [DOI] [PubMed] [Google Scholar]
- Kang S-Y, Lee J-U, Kim K-W. Biosorption of Cr(III) and Cr(VI) onto the cell surface of Pseudomonas aeruginosa. Biochem Eng J. 2007;36:54–58. doi: 10.1016/j.bej.2006.06.005. [DOI] [Google Scholar]
- Khan Z, Hussain SZ, Rehman A, Zulfiqar S, Shakoori A. Evaluation of cadmium resistant bacterium, Klebsiella pneumoniae, isolated from industrial wastewater for its potential use to bioremediate environmental cadmium. Pak J Zool. 2015;47:1533–1543. [Google Scholar]
- Klaassen CD, Liu J, Diwan BA. Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol. 2009;238:215–220. doi: 10.1016/j.taap.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan TA, Chan GY, Lo W-H, Babel S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J. 2006;118:83–98. doi: 10.1016/j.cej.2006.01.015. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesmana SO, Febriana N, Soetaredjo FE, Sunarso J, Ismadji S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem Eng J. 2009;44:19–41. doi: 10.1016/j.bej.2008.12.009. [DOI] [Google Scholar]
- Liu Y, Xiao T, Ning Z, Li H, Tang J, Zhou G. High cadmium concentration in soil in the Three Gorges region: geogenic source and potential bioavailability. Appl Geochem. 2013;37:149–156. doi: 10.1016/j.apgeochem.2013.07.022. [DOI] [Google Scholar]
- Lu W-B, Shi J-J, Wang C-H, Chang J-S. Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp. J1 possessing high heavy-metal resistance. J Hazard Mater. 2006;134:80–86. doi: 10.1016/j.jhazmat.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Martelli A, Rousselet E, Dycke C, Bouron A, Moulis J-M. Cadmium toxicity in animal cells by interference with essential metals. Biochimie. 2006;88:1807–1814. doi: 10.1016/j.biochi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Martins RJ, Pardo R, Boaventura RA. Cadmium (II) and zinc (II) adsorption by the aquatic moss Fontinalis antipyretica: effect of temperature, pH and water hardness. Water Res. 2004;38:693–699. doi: 10.1016/j.watres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Maynaud G, Brunel B, Yashiro E, Mergeay M, Cleyet-Marel JC, Le Quere A. CadA of Mesorhizobium metallidurans isolated from a zinc-rich mining soil is a P(IB-2)-type ATPase involved in cadmium and zinc resistance. Res Microbiol. 2014;165:175–189. doi: 10.1016/j.resmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Naja G, Vanessa M, Volesky B. Biosorption, metal, encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. New York: Wiley; 2010. [Google Scholar]
- Naz N, Young HK, Ahmed N, Gadd GM. Cadmium accumulation and DNA homology with metal resistance genes in sulfate-reducing bacteria. Appl Environ Microbiol. 2005;71:4610–4618. doi: 10.1128/AEM.71.8.4610-4618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333:134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Özdemir S, Kilinc E, Poli A, Nicolaus B, Güven K. Biosorption of Cd, Cu, Ni, Mn and Zn from aqueous solutions by thermophilic bacteria, Geobacillus toebii sub. sp. decanicus and Geobacillus thermoleovorans sub. sp. stromboliensis: equilibrium, kinetic and thermodynamic studies. Chem Eng J. 2009;152:195–206. doi: 10.1016/j.cej.2009.04.041. [DOI] [Google Scholar]
- Palmiter RD. The elusive function of metallothioneins. Proc Natl Acad Sci. 1998;95:8428–8430. doi: 10.1073/pnas.95.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SJ, Chorover J. ATR-FTIR spectroscopy reveals bond formation during bacterial adhesion to iron oxide. Langmuir. 2006;22:8492–8500. doi: 10.1021/la061359p. [DOI] [PubMed] [Google Scholar]
- Penninckx MJ. An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res. 2002;2:295–305. doi: 10.1016/S1567-1356(02)00081-8. [DOI] [PubMed] [Google Scholar]
- Rehman A, Ali A, Muneer B, Shakoori AR. Resistance and biosorption of mercury by bacteria isolated from industrial effluents. Pak J Zool. 2007;39:137–146. [Google Scholar]
- Sari A, Mendil D, Tuzen M, Soylak M. Biosorption of Cd (II) and Cr(III) from aqueous solution by moss (Hylocomium splendens) biomass: equilibrium, kinetic and thermodynamic studies. Chem Eng J. 2008;144:1–9. doi: 10.1016/j.cej.2007.12.020. [DOI] [Google Scholar]
- Say R, Denizli A, Arca MY. Biosorption of cadmium (II), lead (II) and copper (II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour Technol. 2001;76:67–70. doi: 10.1016/S0960-8524(00)00071-7. [DOI] [PubMed] [Google Scholar]
- Shamim S, Rehman A. Antioxidative enzyme profiling and biosorption ability of Cupriavidus metallidurans CH34 and Pseudomonas putida mt2 under cadmium stress. J Basic Microbiol. 2015;55:374–381. doi: 10.1002/jobm.201300038. [DOI] [PubMed] [Google Scholar]
- Sinha S, Mukherjee SK. Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol. 2008;56:55–60. doi: 10.1007/s00284-007-9038-z. [DOI] [PubMed] [Google Scholar]
- Srinath T, Verma T, Ramteke P, Garg S. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere. 2002;48:427–435. doi: 10.1016/S0045-6535(02)00089-9. [DOI] [PubMed] [Google Scholar]
- Srivastava N, Majumder C. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater. 2008;151:1–8. doi: 10.1016/j.jhazmat.2007.09.101. [DOI] [PubMed] [Google Scholar]
- Sulaymon A, Abbood D, Ali A. Competitive adsorption of phenol and lead from synthetic wastewater onto granular activated carbon. J Environ Sci Eng. 2011;5:1389–1399. [Google Scholar]
- Turner JS, Robinson NJ, Gupta A. Construction of Zn2+/Cd2+-tolerant cynadobacteria with a modified metallothionein divergon: further analysis of the function and regulation of smt. J Indust Microbiol. 1995;14:259–264. doi: 10.1007/BF01569937. [DOI] [PubMed] [Google Scholar]
- Vargas-García MDC, López MJ, Suárez-Estrella F, Moreno J. Compost as a source of microbial isolates for the bioremediation of heavy metals: in vitro selection. Sci Total Environ. 2012;431:62–67. doi: 10.1016/j.scitotenv.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Vinodini N, Chatterjee PK, Chatterjee P, Chakraborti S, Nayanatara A, Bhat RM, Rashmi K, Suman V, Shetty SB, Pai SR. Protective role of aqueous leaf extract of Moringa oleiferaon blood parameters in cadmium exposed adult wistar albino rats. Inter J Curr Res Acad Rev. 2015;3:192–199. [Google Scholar]
- Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/S0300-483X(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Xu M, Hadi P, Chen G, Mckay G. Removal of cadmium ions from wastewater using innovative electronic waste-derived material. J Hazard Mater. 2014;273:118–123. doi: 10.1016/j.jhazmat.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Yazdankhah A, Moradi S, Amirmahmoodi S, Abbasian M, Shoja SE. Enhanced sorption of cadmium ion on highly ordered nanoporous carbon by using different surfactant modification. Microporous Mesoporous Mater. 2010;133:45–53. doi: 10.1016/j.micromeso.2010.04.012. [DOI] [Google Scholar]
- Zeng X, Tang J, Liu X, Jiang P. Response of P. aeruginosa E1 gene expression to cadmium stress. Curr Microbiol. 2012;65:799–804. doi: 10.1007/s00284-012-0224-2. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Yin R, Yu L, Wang G, Tian F, Yu R, Zhao J, Liu X, Chen YQ, Zhang H. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. 2015;54:23–30. doi: 10.1016/j.foodcont.2015.01.037. [DOI] [Google Scholar]
- Zhou W, Wang J, Shen B, Hou W, Zhang Y. Biosorption of copper (II) and cadmium (II) by a novel exopolysaccharide secreted from deep-sea mesophilic bacterium. Colloids Surf B Biointerf. 2009;72:295–302. doi: 10.1016/j.colsurfb.2009.04.018. [DOI] [PubMed] [Google Scholar]


