FIGURE 2.
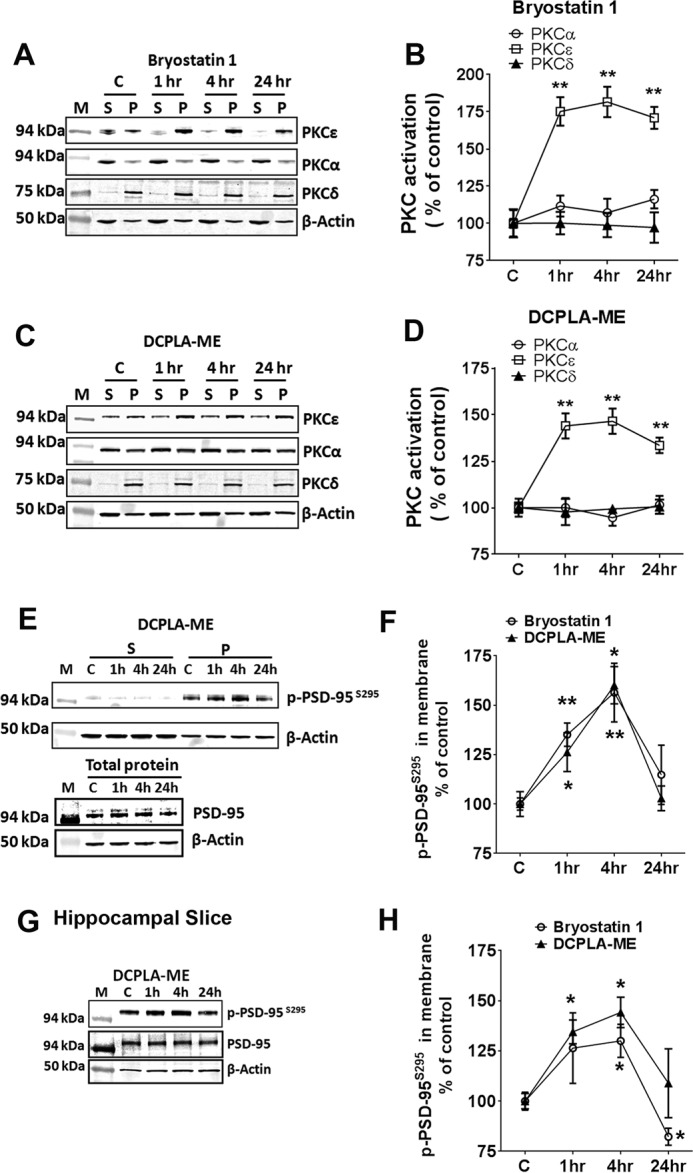
PKCϵ activation induces membrane accumulation of p-PSD-95S295. Primary human neurons were treated with ethanol (C), bryostatin 1 (0.27 nm), or DCPLA-ME (100 nm) for 1. 4, and 24 h. Neurons were separated into soluble (S) and membrane (P) fractions and immunoblotted against PKCα, PKCϵ, and PKCδ. A–C, and p-PSD-95S295, PSD-95 and β-actin. E, M represents molecular weight markers. PKC activation is reported as the percentage of total protein in the membrane. Bryostatin 1-treated neurons showed PKCϵ activation at 1 h (175.2 ± 9.5%; p = 0.002), 4 h (181.6 ± 10.2%; p = 0.0016), and 24 h (170.9 ± 7.4%; p = 0.001) compared with the untreated neurons (100.0 ± 3.1%; F(3,8) = 22.5; ANOVA, p < 0.0005) (B), and DCPLA-ME treated neurons showed a significant increase in PKCϵ activation at 1 h (144.0 ± 6.8%;p = 0.004), 4 h (146.5 ± 6.8%; p = 0.003), and 24 h (133.6 ± 4.2%; p = 0.003) compared with the untreated neurons (F(3,8) = 15.7; ANOVA, p = 0.001). D, induced PKCϵ activation at 1, 4, and 24 h. PKCϵ activation significantly increased the p-PSD-95S295 expression in the membranes at 1 h and 4 h. F, adult rat hippocampal organotypic slices were treated with ethanol (C), bryostatin 1 (0.27 nm), or DCPLA-ME (100 nm) for 1, 4, and 24 h. Protein lysates were immunoblotted against p-PSD-95S295, PSD-95, and β-actin. G, PKCϵ activation significantly increased the p-PSD-95S295 expression in the membranes at 1 and 4 h. H, data are represented as the mean ± S.E. of three independent experiments (Student's t test. *, p < 0.05; **, p < 0.005).
