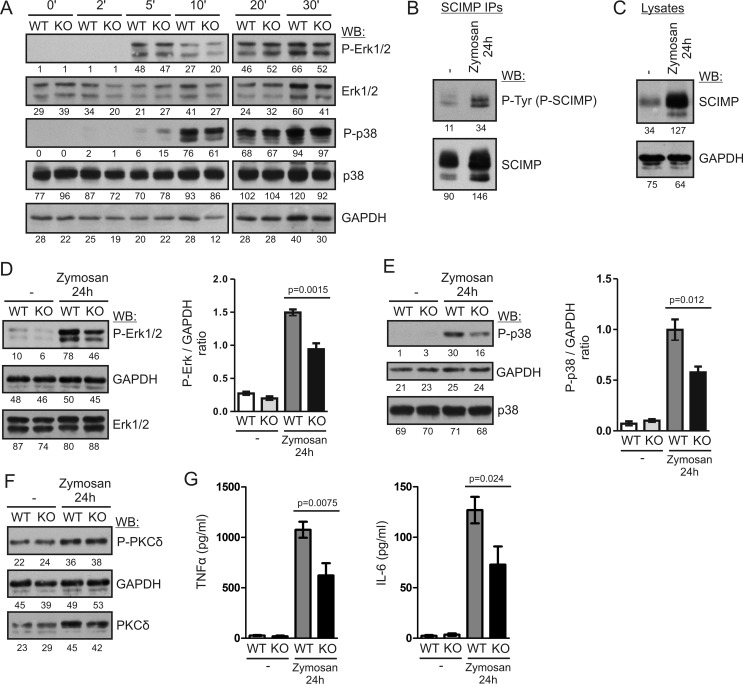
FIGURE 6.
SCIMP enhances long-term MAP kinase activation and pro-inflammatory cytokine production after stimulation with zymosan. A, BMDCs from WT and Scimp−/− (KO) mice were stimulated with 300 μg/ml zymosan for the indicated times, and ERK1/2 and p38 phosphorylation was analyzed by immunoblotting. WB, Western blotting. B, BMDCs from C57Bl/6J mice were stimulated with 300 μg/ml zymosan for 24 h or left unstimulated, lysed, and subjected to SCIMP immunoprecipitation followed by immunoblotting with phosphotyrosine (P-Tyr) and SCIMP antibodies. C, levels of SCIMP protein in the lysates of BMDCs stimulated with 300 μg/ml zymosan for 24 h or left unstimulated were analyzed by immunoblotting with SCIMP antibodies. D, phosphorylation of ERK1/2 in the lysates of WT and Scimp−/− BMDCs stimulated with zymosan for 24 h or left unstimulated (left panel). The right panel shows the results of densitometric quantification of ERK phosphorylation in samples prepared from 4 mice/genotype. E, a similar analysis of p38 phosphorylation in the samples from D. F, a similar analysis of PKCδ phosphorylation. G, late production of TNFα and IL-6 by WT and Scimp−/− BMDCs. BMDCs were stimulated with 300 μg/ml zymosan for 48 h, washed, and cultured for an additional 24 h in the absence of stimulus. After this period, culture supernatants were collected and analyzed by ELISA for the presence of TNFα and IL-6. In the experiments shown in B–G, mice with a MyD88-deficient genetic background were used.