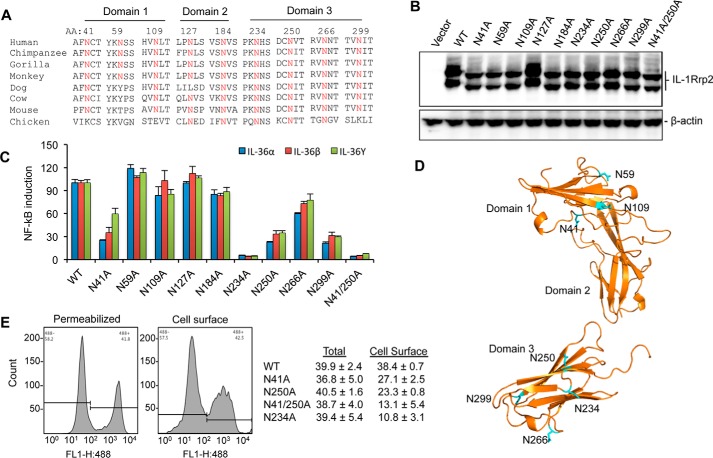
FIGURE 5.
N-linked glycosylation in IL-1Rrp2 is critical for signaling and trafficking to the cell surface. A, sequence alignment of predicted N-linked glycosylation sites in IL-1Rrp2 and orthologs from other species. Residues in IL-1Rrp2 predicted to be glycosylated are shown above the IL-1Rrp2 sequence. B, effects of mutations in the predicted N-linked glycosylation residues on the electrophoretic migration of the resultant IL-1Rrp2 proteins. C, effects of substitution of predicted glycosylation sites in the IL-1Rrp2 on signal transduction by all three IL-36 cytokines. 293T cells were transfected with equal amounts of the plasmids expressing WT or mutant IL-1Rrp2. The cells were mock-treated or stimulated with 2 ng/ml of the IL-36 agonists. The data were plotted relative to WT IL-1Rrp2 from two independent experiments, each performed with three independent samples. D, model of the localization of glycosylation sites in the IL-1Rrp2 ECD. The asparagine residues are marked in blue. E, cellular location of WT or mutant IL-1Rrp2 was determined by flow cytometry. Total amount of IL-1Rrp2 was determined in detergent-permeabilized cells while IL-1Rrp2 present on the cell surface was detected in non-permeablilized cells. Flow cytometry was performed as described in “Experimental Procedures.” The data were presented as averages of four independent samples.