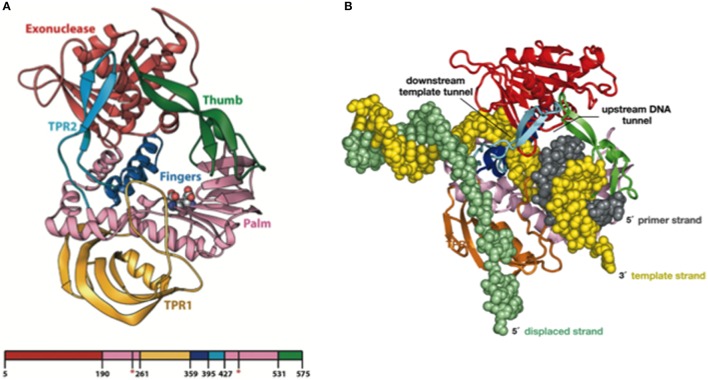
Figure 3.
(A) Ribbon Representation of the Domain Organization of Φ29 DNAP. The exonuclease domain is shown in red, the palm in pink, TPR1 in gold, the fingers in blue, TPR2 in cyan, and the thumb in green. Asp249 and Asp458, which provide the catalytic carboxylates of the polymerase active site, are shown using space-filling spheres. Reproduced with permission from Kamtekar et al. (2004). (B) Modeling processivity and strand displacement in Φ29 DNAP. The TPR2 insertion would contribute to a full encirclement of the DNA substrate, conferring a remarkable processivity, and also acts as a structural barrier, which would force the DNA strands of the parental DNA to diverge (melt). Because Φ29 DNAP translocates after each polymerization cycle, the TPR2 subdomain would act as a wedge to couple polymerization to strand displacement. Reproduced with permission from Rodríguez et al. (2005). Copyright (2005) National Academy of Sciences, U.S.A.