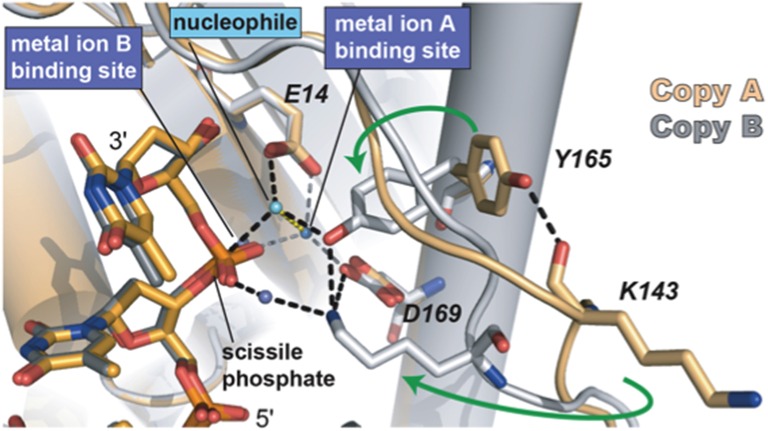
Figure 5.
The two conformations of the exonuclease active site of Φ29 DNAP. The Lys143, Tyr165, and two of the catalytic aspartates are shown in stick representation. Green arrows indicate the movement of Tyr165 and Lys143 from the open conformation to the closed conformation. The black dashed lines represent the observed hydrogen bonds between Lys143 and Tyr165 with each other and with other parts of the active site. The interactions between the waters in the metal binding sites and the protein are represented as gray dashes. Most of the interactions that the water in the metal in B site would be making with the protein are missing due to the D12A/D66A mutations in the polymerase used in these crystallographic studies. Reproduced with permission from Berman et al. (2007).