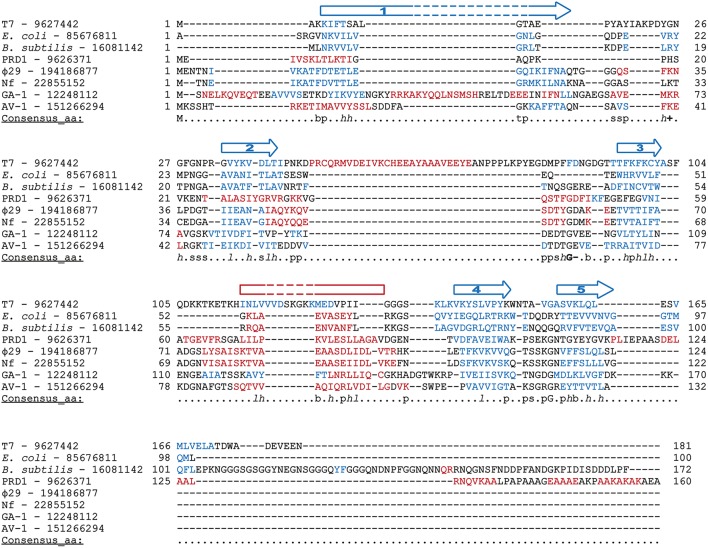
Figure 9.
Multiple sequence alignment of diverse SSBs from prokaryotic origin. Source and GenBank identification number (GI) of each protein is indicated. Alignment was made with Promals3D (Pei et al., 2008), based secondary structure predictions and the crystal structure of E. coli and T7 SSBs (1SRU and 1JE5, respectively, in Protein Data Bank). The protein sequences are colored according to actual or predicted secondary structures (red: alpha-helix, blue: beta-strand). Also, the consensus five beta-strands and the alpha-helix that correspond with a common OB-fold are depicted above the sequences. Note that in the case of T7 SSB the α-helix is between the second and third strands. The last line in each block (Consensus_aa) shows consensus amino acid sequence as follows: conserved amino acids are in uppercase letters; aliphatic (I, V, L): l; aromatic (Y, H, W, F): @; hydrophobic (W, F, Y, M, L, I, V, A, C, T, H): h; alcohol (S, T): o; polar residues (D, E, H, K, N, Q, R, S, T): p; tiny (A, G, C, S): t; small (A, G, C, S, V, N, D, T, P): s; bulky residues (E, F, I, K, L, M, Q, R, W, Y): b; positively charged (K, R, H): +; negatively charged (D, E): −; charged (D, E, K, R, H): c.