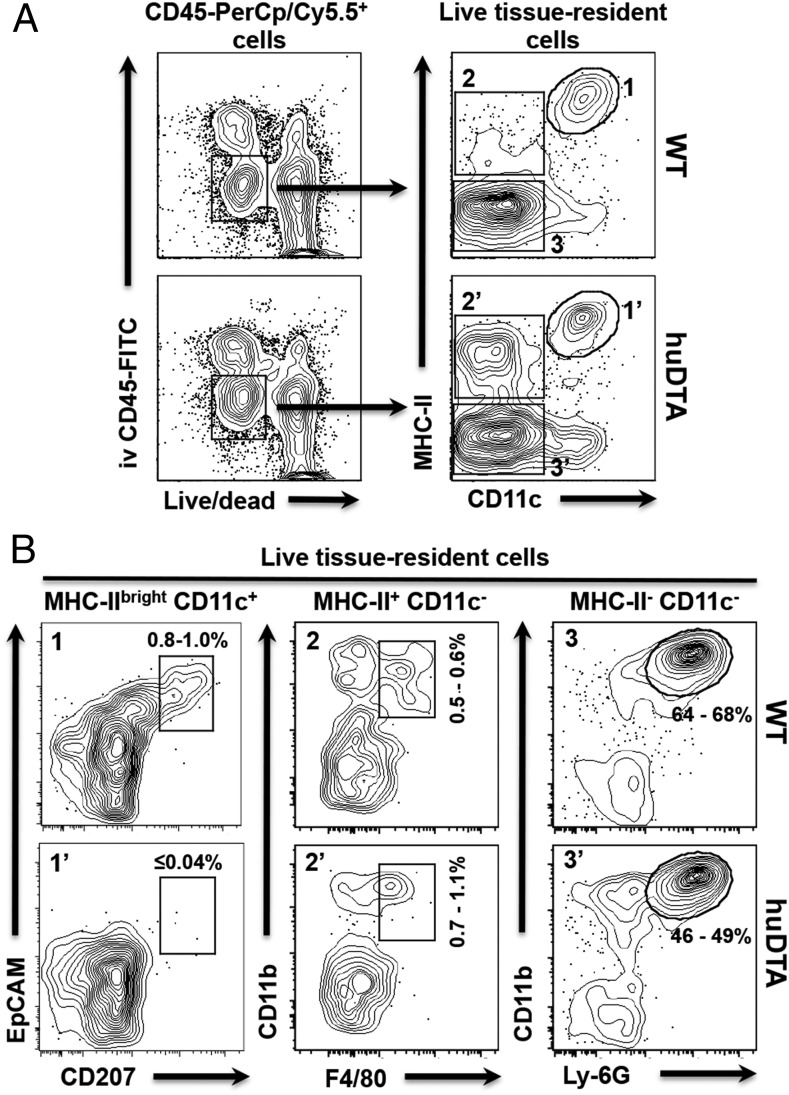
FIGURE 2.
LCs do not repopulate the oral mucosa of huLangerin-DTA (huDTA) mice following sustained oral colonization with P. gingivalis. Groups of 6- to 8-wk-old huDTA and C57BL/6J (wild-type [WT]) mice were inoculated six times with P. gingivalis strain 53977 by oral gavage at 4-d intervals. Mice were injected retro-orbitally with a nonsaturating amount of FITC-conjugated anti-mouse CD45 mAb (1.25 μg) 3 min prior to sacrifice to allow FITC-stained blood-resident immune cells to be excluded from downstream flow cytometry analysis. Single-cell suspensions prepared from pooled (two mice) oral mucosal tissue were stained with cell Live/Dead discriminating dye followed by anti-mouse CD45, CD11b, CD11c, I-Ab (MHC-II), EpCAM, CD207, F4/80, and Ly-6G flurochrome-conjugated mAbs and analyzed by flow cytometry. (A) Flow cytometry gating strategy used to identify live tissue-resident immune cells. Representative dot plots for pooled WT and huDTA mouse samples are shown. Initial gates were drawn around CD45-PerCP/Cy5.5+ cells that were alive (low fluorescence dye staining) and residing within the tissue (i.v. CD45-FITC−). Cells residing in the vasculature are CD45-FITC+ and are not included in these gates. The live, tissue-resident immune cells were divided into three distinct populations based on expression of MHC-II and CD11c (MHC-IIbrightCD11c+, gates 1 and 1′; MHC-II+CD11c−, gates 2 and 2′; and MHC-II−CD11c−, gates 3 and 3′). (B) Representative dot plots for WT and huDTA mouse samples are shown with gates drawn around LCs (EpCAM+CD207+; panels 1 and 1′), macrophages (CD11b+F4/80+; panels 2 and 2′), and neutrophils (CD11b+Ly-6G+; panels 3 and 3′) derived from their respective parental gates. The number of cells within each of the drawn gates is expressed as a percentage of the total live tissue-resident immune cell population and indicates the range found among all samples analyzed.