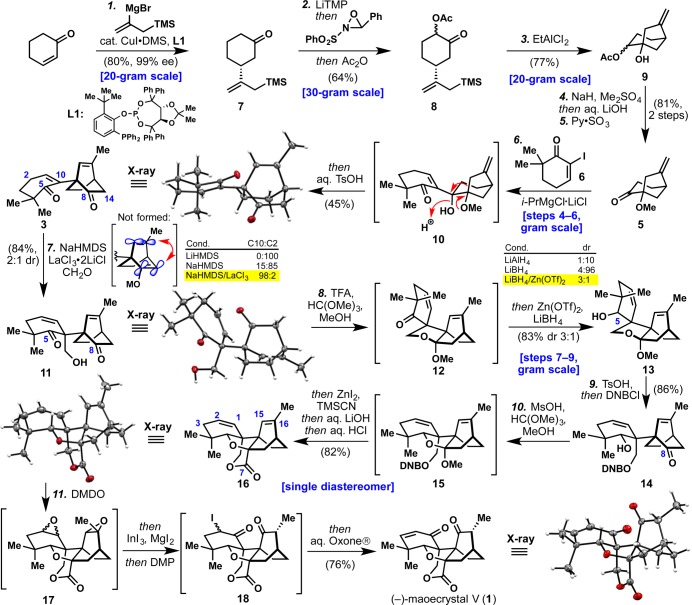
Scheme 1. Total Synthesis of (−)-Maoecrystal V (1).
Reagents and conditions: (1) CuI·0.75DMS (0.60 mol %), L1 (0.80 mol %), TMSCH2C(MgBr)CH2 (2.5 equiv), PhMe/MeTHF, −78 °C (80%, 99% ee); (2) LiTMP (1.02 equiv), THF, −78 °C; then Davis oxaziridine (1.3 equiv), THF, DMPU, −78 °C; then Ac2O (1.2 equiv), −78 to 0 °C (64%); (3) EtAlCl2 (2.0 equiv), PhMe, 0 °C (77%); (4) NaH (1.2 equiv), Bu4NI (1.0 equiv), Me2SO4 (3.0 equiv), DMF, 23 °C; then aq. LiOH (8.5 equiv), 23 °C; (5) Py·SO3 (3.5 equiv), Et3N (8.5 equiv), DMSO, DCM, 0 to 23 °C (81%, 2 steps); (6) i-PrMgCl·LiCl (1.5 equiv), 6 (1.5 equiv), PhMe, −78 to 0 °C; then aq. TsOH (6.5 equiv), 0 to 85 °C (45%); (7) NaHMDS (1.3 equiv), LaCl3·2LiCl (1.0 equiv), THF, DMPU, CH2O(g) (10 equiv), −45 °C (84%, 2:1 dr); (8) TFA (0.50 equiv), HC(OMe)3, MeOH, 60 °C; then Zn(OTf)2 (2.0 equiv), LiBH4 (8.5 equiv), DCM, 23 °C (83%, 3:1 dr); (9) TsOH (5.0 mol %), H2O (2.0 equiv), THF, 23 °C; then DNBCl (3.5 equiv), DMAP (0.20 equiv), Et3N (5.0 equiv), 23 °C (86%); (10) MsOH (5 × 0.50 equiv), HC(OMe)3, MeOH, 65 °C; then ZnI2 (0.30 equiv), TMSCN (5.0 equiv), 23 °C; then aq. LiOH (12 equiv); then aq. HCl (20 equiv), 65 °C (82%); (11) DMDO (2 × 3.0 equiv), acetone, 23 °C; then InI3 (5.0 mol %), MgI2 (1.2 equiv), MeCN, 23 °C; then DMP (3.0 equiv), 23 °C; then aq. Oxone (10 equiv), Bu4NHSO4 (0.10 equiv), pH = 7.4 buffer, 23 °C (76%); DMS = dimethyl sulfide, MeTHF = 2-methyltetrahydrofuran, LiTMP = lithium 2,2,6,6-tetramethylpiperidide, DMPU = N,N′-dimethylpropyleneurea, DMSO = dimethyl sulfoxide, DCM = dichloromethane, TsOH = p-toluenesulfonic acid, NaHMDS = sodium bis(trimethylsilyl)amide, TFA = trifluoroacetic acid, DNBCl = 3,5-dinitrobenzoyl chloride, MsOH = methanesulfonic acid, DMDO = dimethyldioxirane, DMP = Dess–Martin periodinane.