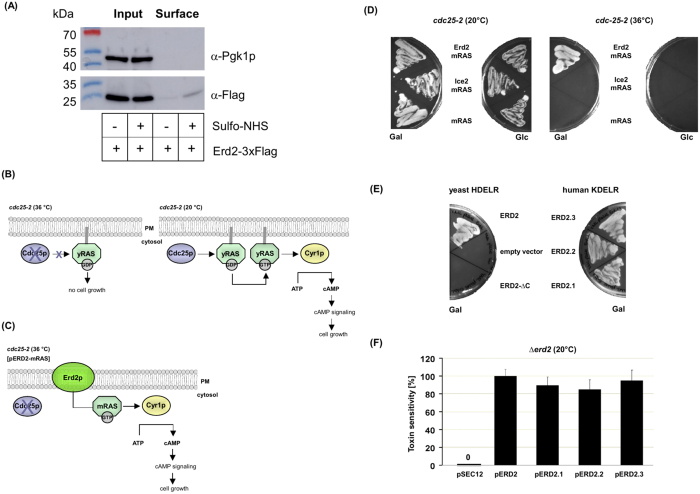
Figure 5.
(A) Cell surface biotinylation of yeast Erd2p. Wild-type cells expressing Erd2p-3xFlag from its endogenous promoter and chromosomal locus were biotinylated by treatment with (+) or without (−) Sulfo-NHS-SS-Biotin and purified with avidin beads. Whole cell lysates (input) served as control to determine the total amount of Erd2p (detected with anti-Flag), while phosphoglycerate kinase (Pgk1p) served as cytosolic marker protein to determine cellular integrity of the samples. Membrane fraction (surface) represents the pool of Erd2p at the cell surface (note that the faint signal in the surface fraction without Sulfo-NHS treatment results from unspecific binding of Erd2-3xFlag to the avidin-coupled agarose beads). (B,C) Schematic outline of the RAS recruitment system. Expression of a constitutively active cytosolic variant of mammalian RAS (mRAS) lacking a farnesyl membrane anchor activates adenylate cyclase (Cyr1p) and restores cell growth of a cdc25-2ts mutant only when fused to a membrane protein vehicle (Erd2p) that ensures its delivery to the plasma membrane. (D) Growth phenotype of a yeast cdc25-2ts mutant expressing cytosolic mRAS or mRAS fusions to the cytosolic C-termini of Ice2p (Ice2p-mRAS) or Erd2p (Erd2p-mRAS). (E) Human KDEL receptors complement Erd2p function in yeast. Growth of Δerd2 cells expressing either of the three human KDEL receptors (ERD2.1, ERD2.2, ERD2.3), a full-length (ERD2) or a C-terminal truncated and, thus, non-functional yeast H/KDEL receptor (ERD2-∆C), or empty vector control cells monitored under inducing conditions after 5 d at 30 °C. (F) Human KDEL receptors restore K28 toxin sensitivity in a yeast Δerd2 null mutant. Sensitivity of Δerd2 cells expressing yeast Erd2p was set 100%. Mean average (n = 10) and standard deviation is displayed. For p-value calculation, cells expressing Erd2p were compared to cells expressing the indicated mammalian KDEL receptor.