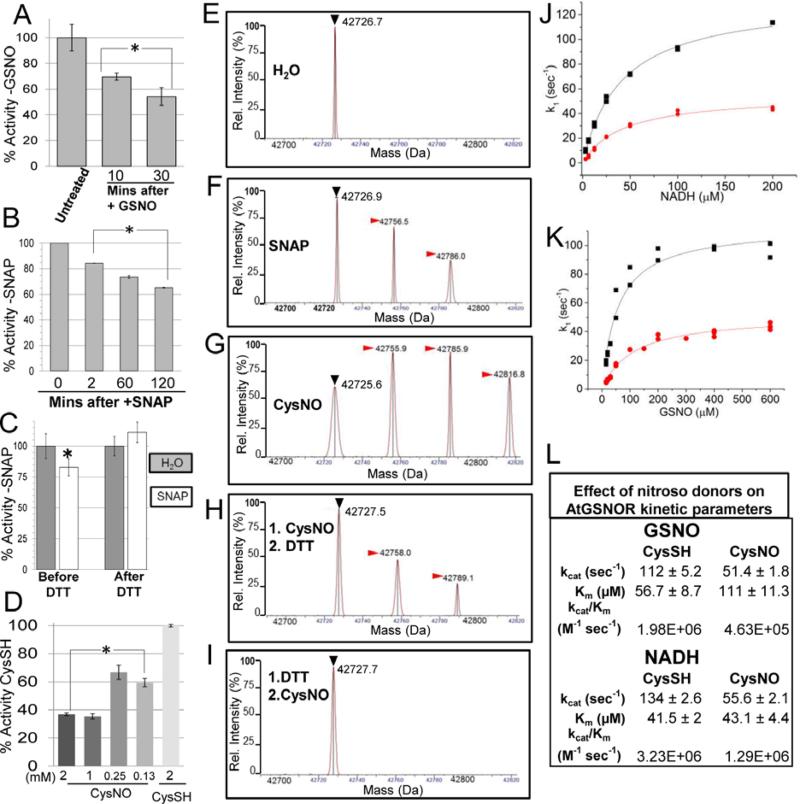
Figure 1. Modification of Arabidopsis GSNOR with reactive nitrogen donors inhibits enzyme activity and is reversed by DTT in vitro.
A. AtGSNOR activity decreases when incubated with the substrate and nitrosating agent GSNO. B. AtGSNOR activity decreases when incubated with the nitroso donor SNAP. C. Inhibition of AtGSNOR by SNAP is reversed by the reducing agent DTT. Before DTT: AtGSNOR was incubated with SNAP or water for 30 minutes prior to assessing enzyme activity. After DTT: SNAP- or water-treated protein solutions were brought to 5mM DTT and incubated for 5 minutes prior to assessing enzyme activity. D. Concentration dependence of CysNO-induced inhibition of AtGSNOR. Enzymes were assayed in triplicate (A-C) or quadruplicate (D). Error bars signify two times standard error. Asterisks indicatestatistically significant differences for a treatment relative to an initial time point or condition. E-I: Deconvolved mass spectra of AtGSNOR treated with water (D), SNAP (E), the NO donor CysNO alone (F), followed by DTT (G), or preceded by DTT (H). Black (▶) and red ( ) arrowheads designate unmodified GSNOR and nitrosated GSNOR adducts (+29 Da), respectively. J-L: CysNO treatment reduces the enzymatic efficiency of AtGSNOR. CysSH- (black) and CysNO- (red) treated AtGSNOR were assayed at saturating concentrations of NADH (J) or GSNO (K) over a range of concentrations of the corresponding substrate. L. Summary of kinetic parameters, plus or minus twice the standard error.
) arrowheads designate unmodified GSNOR and nitrosated GSNOR adducts (+29 Da), respectively. J-L: CysNO treatment reduces the enzymatic efficiency of AtGSNOR. CysSH- (black) and CysNO- (red) treated AtGSNOR were assayed at saturating concentrations of NADH (J) or GSNO (K) over a range of concentrations of the corresponding substrate. L. Summary of kinetic parameters, plus or minus twice the standard error.