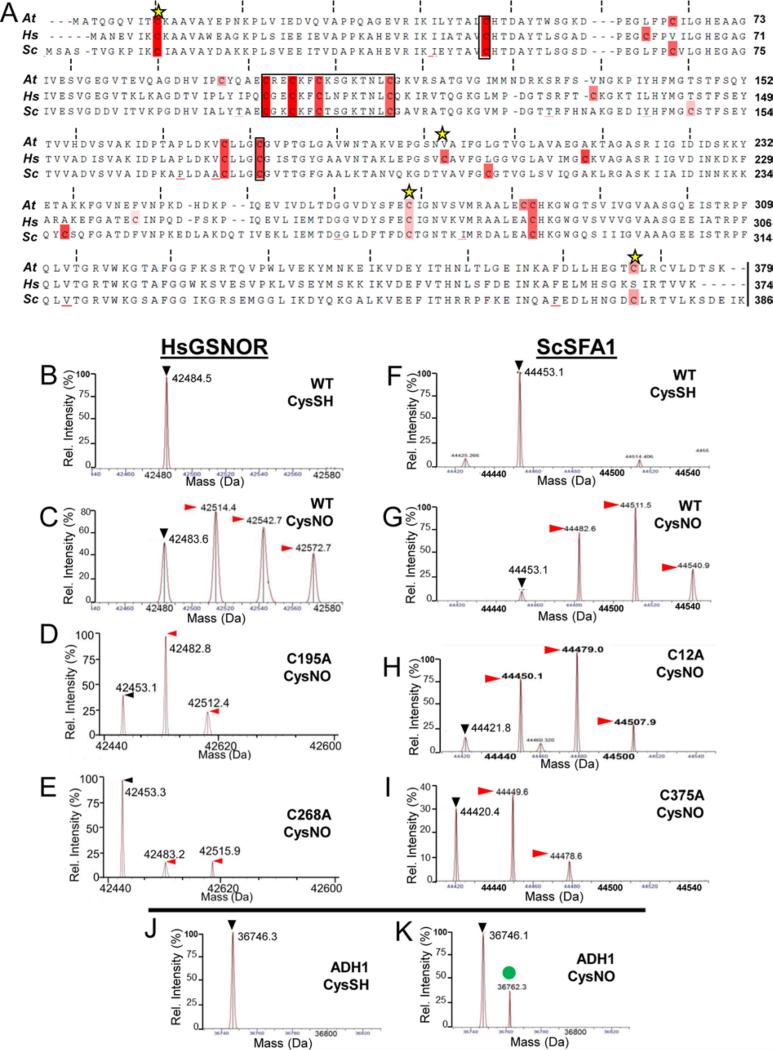
Figure 3. Nitrosation of human and yeast GSNORs also depends on conserved cysteine residues.
A. Multiple sequence alignment of GSNORs examined in this study. Black boxes denote zinc-coordinating cysteines, while yellow stars indicate putative nitrosation sites according to GPS SNO [34]. B-I: Deconvolved mass spectra of wild-type and cysteine mutant human GSNOR (B-E) and the yeast ortholog SFA1 (F-I) treated with the NO donor CysNO. Black (▶) and red ( ) arrowheads designate unmodified GSNOR and nitrosated GSNOR adducts (+29 Da), respectively. J-K: Yeast alcohol dehydrogenase 1 (ADH1) is not nitrosated under the same conditions. The green circle (
) arrowheads designate unmodified GSNOR and nitrosated GSNOR adducts (+29 Da), respectively. J-K: Yeast alcohol dehydrogenase 1 (ADH1) is not nitrosated under the same conditions. The green circle ( ) demarcates an unknown 16 Da adduct in the CysNO-treated sample.
) demarcates an unknown 16 Da adduct in the CysNO-treated sample.