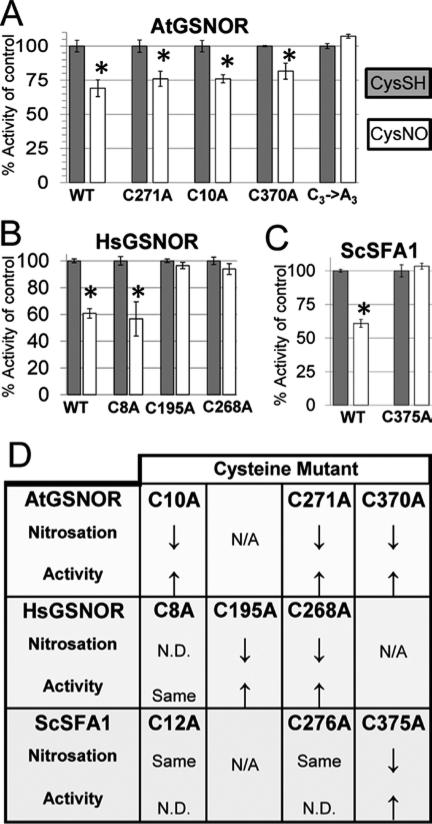
Figure 4. Nitrosation-induced inhibition of GSNOR enzyme activity is blocked by mutation of conserved cysteine residues.
Arabidopsis (A), human (B), and yeast (C) wild-type and cysteine mutant GSNOR isoforms were treated with CysNO or CysSH as a control, after which proteins were buffer exchanged with 20mM Tris pH 8 and assayed as described in triplicate. Error bars signify two times standard error. Asterisks indicate statistically significant differences in catalysis upon CysNO treatment of each protein relative to its CysSH control. D: Summary of the effect of replacement of single conserved cysteines on nitrosation and nitrosation-induced enzyme activity inhibition. Up- and down-arrows are relative to wild-type. N/A: Not applicable (there is no homologous cysteine at this position ). N.D.: Not determined.