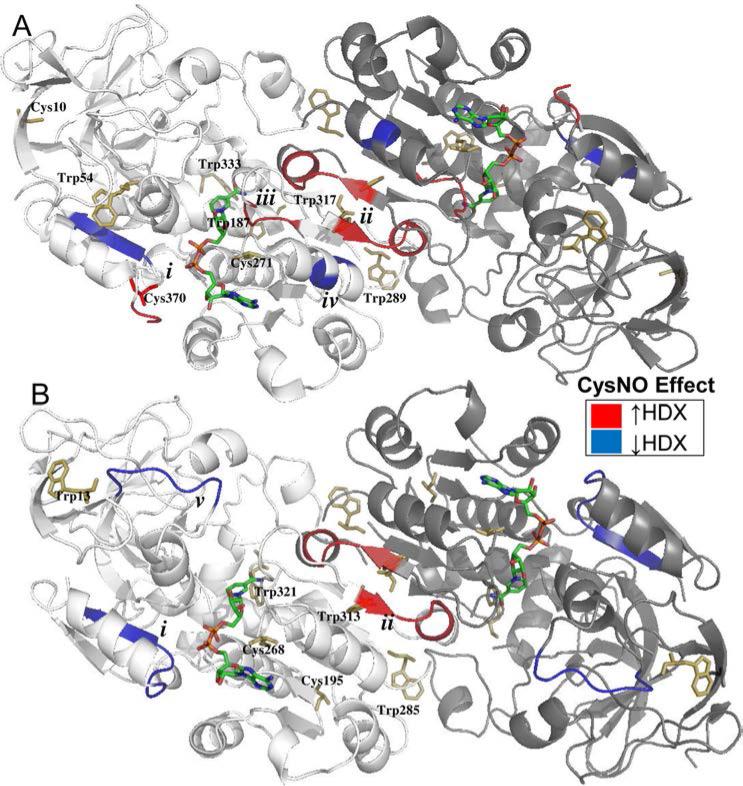
Figure 5. Nitrosation affects the hydrogen-deuterium exchange of similar conserved regions of Arabidopsis and Human GSNOR.
A-B: X-ray structure cartoons of Arabidopsis (A, PDB 3UKO) and Human (B, PDB 1MP0) GSNOR co-crystalized with NADH (multicolored sticks). One monomer in each dimer is shaded more darkly for clarity. Tryptophans and predicted nitrosated cysteines are depicted with yellow sticks. Relative to the CysSH-treated control, CysNO induced similar regions of both proteins to exhibit decreased (blue) or increased (red) hydrogen-deuterium exchange. A. AtGSNOR. i. Glu367-Leu375, contains cysteine 370 and located 5Å from residues that bind NADH. ii. Ser305-Gln310, comprises part of the dimer interface. iii. Ile294-Ala298, contains NADH-binding residues and located 5Å from cysteine 271. iv. Ala280-Leu282, located 6 Å from (iii). B. HsGSNOR. i. Gly365-Val372, located 5Å from residues that bind NADH, homologous to (i) in AtGSNOR. ii. Ala301-Gln306, comprises part of the dimer interface, homologous to (ii) in AtGSNOR. v. Pro57-Val63, constitutes part of the roof of the substrate-binding cavity.