Abstract
Despite significant improvements in preoperative patient evaluation and surgical planning, vascular access failure in patients on hemodialysis remains a frequent and often unforeseeable complication. Our inability to prevent this complication is, in part, because of an incomplete understanding of how preexisting venous and arterial conditions influence the function of newly created arteriovenous fistulas and grafts. This article reviews the relationship between three preexisting vascular pathologies associated with CKD (intimal hyperplasia, vascular calcification, and medial fibrosis) and hemodialysis access outcomes. The published literature indicates that the pathogenesis of vascular access failure is multifactorial and not determined by any of these pathologies individually. Keeping this observation in mind should help focus our research on the true causes responsible for vascular access failure and the much needed therapies to prevent it.
Keywords: arteriovenous fistula, arteriovenous graft, pre-existing pathologies, vascular calcification, vascular fibrosis, intimal hyperplasia, arteries, Humans, Hyperplasia, renal dialysis, Renal Insufficiency, Chronic, Veins
Introduction
Preventing vascular access failure in patients on hemodialysis remains a challenge. Despite ongoing efforts to enhance preoperative patient evaluation and surgical planning, the primary access failure (inability to ever use it for dialysis) and primary unassisted patency (access remaining functional without an intervention) rates of arteriovenous fistulas (AVFs) and arteriovenous grafts (AVGs) remain unsatisfactory. The frequency of primary failure in AVFs, the vascular access of choice, is approximately 40% (1), whereas the primary unassisted patency is about 60% at 1 year after creation (2). In contrast, primary failure occurs in only approximately 20% of AVGs (3,4), but this advantage over AVFs is offset by a lower 1-year primary patency rate (30%–50%) and a threefold higher frequency of interventions to maintain functionality for dialysis (3).
At present, salvage of failed AVFs and AVGs is almost exclusively achieved by the use of endovascular and surgical interventions. In the absence of more effective therapeutic options to prevent access failure, this approach exposes the patients to the inconvenience and risks of additional procedures and substantially increases the costs associated with the maintenance of the vascular access (5). Despite the magnitude of this medical problem, there have been no major novel therapeutic interventions in the field of hemodialysis access for the past few decades. This stagnation likely reflects our incomplete understanding of how venous and arterial conditions before access creation influence the development and function of AVFs and AVGs.
The creation of a hemodialysis access pushes the boundaries of arterial and venous distensibility to unprecedented limits. In the first few weeks after AVF creation, the blood flow in the brachial artery of patients with successfully mature AVFs increases to 800–1500 ml/min (6) compared with a baseline flow of approximately 100 ml/min in the native artery before AVF creation (7). Similarly, radiocephalic AVFs have average blood flows of 600–1000 ml/min, with mean values being approximately two times higher in brachiocephalic AVFs (8). It is plausible that any preexisting vascular factor that compromises arterial and venous adaptation to these new hemodynamic conditions will be detrimental to hemodialysis access outcomes. Whether preexisting vascular pathologies associated with or aggravated by CKD can impair the necessary adaptive remodeling of blood vessels after vascular access placement is still an open question. This review examines recent advances in our understanding of the effect of preexisting intimal hyperplasia (IH), vascular calcification, and medial fibrosis on vascular access outcomes (Table 1). It also summarizes clinical strategies to improve preoperative vascular health and proposes future research approaches to elucidate the vascular risk factors that predispose to hemodialysis access failure.
Table 1.
Associations between preexisting vascular pathologies and vascular access outcomes according to the published literature
| Reference | Access Type | Sample Type | No. of Patients | Findings | Comments |
|---|---|---|---|---|---|
| Preexisting IH | |||||
| Kim et al. 2003 (14) | AVF | Radial artery (n=59) | 59 | Positive association between IH and 1 yr of primary unassisted patency (P=0.002) | Analysis of circumferential samples is not possible |
| Lee et al. 2011 (13) | AVF (n=7) | Cephalic veins (n=6) | 12 | Positive association between IH and maturation failure in AVF (P=0.03) | Small sample size |
| AVG (n=5) | Axillary veins (n=3), brachial vein (n=1), basilic vein (n=1), antecubital vein (n=1) | ||||
| Allon et al. 2013 (28) | AVF | Forearm veins (n=44), upper arm veins (n=85) | 129 | No association between IH and postoperative stenosis (P=0.49) | The association between IH and maturation failure or patency was not evaluated |
| Allon et al. 2013 (55) | AVG | Arteries (n=73), veins (n=76) | 76 | No association between arterial (P=0.30) or venous IH (P=0.10) and primary unassisted patency; same for cumulative patency (P=0.40 and P=0.20, respectively); high arterial and venous IHs were associated with lower frequency of interventions (P<0.001 for both) | Analysis of circumferential arterial samples is not possible |
| Tabbara et al. 2016 (10) | AVF | Basilic veins (n=55), brachial veins (n=4) | 96 | No association of preexisting IH with postoperative IH (P=0.70) or primary unassisted patency (P=0.20) | Definition of maturation did not take into account cannulation failures |
| Preexisting medial fibrosis | |||||
| Allon et al. 2013 (28) | AVF | Arteries (n=50) | 50 | Arterial medial fibrosis >70% was not associated with maturation failure (P=0.50) | Analysis of circumferential arterial samples is not possible |
| Allon et al. 2013 (55) | AVG | Arteries (n=68) | 76 | Arterial medial fibrosis >70% was not associated with primary unassisted patency (P=0.60) or cumulative patency (P=0.30) but did predict a lower frequency of interventions (P<0.001) | Analysis of circumferential arterial samples is not possible |
| Preexisting vascular calcificationa | |||||
| Lockhart et al. 2004 (56) | AVG | Arteries (n=32) | 32 | Positive association between macrocalcification and technical graft failure (P=0.004) but not with 1 yr of cumulative patency (P=0.09) | Calcification scoring did not include internal iliac arteries |
| Allon et al. 2013 (28) | AVF | Arteries (n=50) | 50 | The presence and score of arterial microcalcification were not associated with maturation failure (P=0.60 and P=0.08, respectively) | Analysis of circumferential arterial samples is not possible |
| Allon et al. 2013 (55) | AVG | Arteries (n=66) | 76 | Arterial microcalcification >10% was not associated with primary unassisted patency (P=0.30) or cumulative patency (P=0.70) but did predict a lower frequency of interventions (P=0.001) | Analysis of circumferential arterial samples is not possible |
| Yun et al. 2014 (52) | AVF | Radial artery (n=98), brachial artery (n=51) | 149 | The presence of microcalcification was associated with increased cardiovascular mortality (P=0.03) | Analysis of circumferential arterial samples is not possible |
| Allon et al. 2015 (50) | AVF | Arteries (n=127) | 127 | Arterial microcalcification was not associated with stenosis (P=0.30), maturation failure (P=0.90), or primary unassisted patency (P=0.20); arterial macrocalcification by ultrasound was also not associated with AVF maturation (P=0.90) | Analysis of circumferential arterial samples is not possible; patients with severe concentric calcification were excluded |
| Choi et al. 2015 (51) | AVF | Radial arteries (n=92), brachial arteries (n=22) | 114 | Positive association between arterial microcalcification and 1 yr primary unassisted patency (P=0.04) | Analysis of circumferential arterial samples is not possible; patients with primary failure within 1 wk of surgery were excluded |
| Little et al. 2015 (57) | AVG | Femoral arteries (n=143) | 143 | Circumferential macrocalcification >50% was associated with primary surgical technical failure (P=0.002) and shorter cumulative patency (P=0.003) |
IH, intimal hyperplasia; AVF, arteriovenous fistula; AVG, arteriovenous graft.
Macrocalcification was determined using radiologic methods, whereas microcalcification was assessed using histopathology.
IH
Pathobiology of Preexisting IH
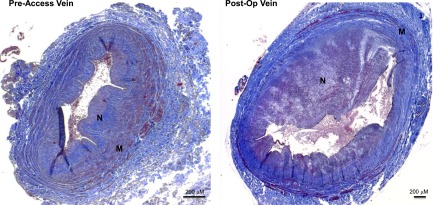
Postoperative IH secondary to the hyperplastic accumulation of α-smooth muscle actin–positive cells in the tunica intima of veins has been historically associated with stenosis and early failure of vascular accesses (9). Although it is unlikely that postoperative IH alone leads to critical AVF occlusion (10), it may be a contributing factor to AVF nonmaturation in the setting of inadequate outward remodeling. More recently, IH has been also identified as a common preexisting pathology in the veins and arteries used for hemodialysis access creation (10–14). Wali et al. (12) observed that, in contrast with cephalic veins from young organ donors with normal kidney function, veins from patients with CKD frequently exhibit focal or diffuse IH populated by myofibroblastic vascular smooth muscle cells (VSMCs) that have irregular shapes and marked vacuolation around the nucleus and along the cell periphery. Two subsequent cohorts confirmed preexisting IH in the veins and arteries of patients with CKD (13,14). These studies emphasized the profound histologic differences between preexisting and postoperative IH (Figure 1). Postoperative lesions are recognized as eccentric concentrations of myofibroblasts in the intima of AVFs and AVGs (9,10,15), whereas the preexisting venous pathology consists of concentric accumulations of both myofibroblasts and contractile VSMCs underneath the endothelial line (10,13). The National Institute of Diabetes and Digestive and Kidney Diseases–funded multicenter, observational Hemodialysis Fistula Maturation (HFM) Study confirmed the high prevalence of preexisting IH in veins used to create an AVF in 370 patients with CKD.
Figure 1.
Preexisting and postoperative intimal hyperplasia in basilic veins collected before creation and superficialization (6 weeks later) of a planned two–stage arteriovenous fistula. Sections were Masson trichrome stained. M, tunica media; N, neointima or intima.
The etiology of preexisting IH in peripheral veins and arteries of patients on hemodialysis remains poorly understood. It is likely that this type of IH, similar to preexisting medial fibrosis and wall calcification, takes place early in the course of renal dysfunction secondary to volume/flow overload (anemia, sodium, and water retention) as well as other poorly defined clinical factors unique to this population. Vascular injury associated with comorbid factors, vascular trauma related to venipuncture, and endothelial dysfunction may also contribute to intimal thickening in the native vessels of patients with CKD. The uremic milieu causes VSMC dedifferentiation and proliferation in vitro and increases IH in animals with CKD (16). A seminal study showed a positive association between the degree of arterial intimal thickness and calcification and the duration of uremia (17). Nonetheless, similar pathologies have been observed in the cephalic and saphenous veins of elderly patients with normal renal function undergoing arterial grafting (18), suggesting that uremia is not the only biologic insult causing vascular intimal thickening in patients with CKD. Tabbara et al. (10) obtained two consecutive basilic vein samples from patients undergoing a planned two–stage AVF: the first sample from the native vein used to create the AVF and the second one several weeks later from the draining vein of the AVF (Figure 1). Patient age, sex, and diabetes were not associated with the maximal intimal thickness of the native basilic vein, IH of the draining vein of the AVF, or the change in venous IH between paired vein samples from the same individual (10). Although vascular trauma from venipuncture and intravenous peripheral lines may contribute to preexisting vascular pathologies, it does not explain the extensive IH frequently found in distal saphenous veins that have never been punctured or traumatized (18).
At present, dedifferentiation and migration of VSMCs into the subendothelial space of veins and arteries as a result of endothelial dysfunction are the most plausible hypotheses for the spontaneous development of IH in native vessels. Preexisting endothelial dysfunction secondary to reduced vascular bioavailability of nitric oxide (NO) is common in patients with CKD and on hemodialysis (19,20). It may impair vasodilation and increase the risk for thrombosis and IH after AVF creation. A recent pilot study showed an association between higher peripheral arterial tonometry, a measure of microvascular endothelial function, and successful AVF outcomes (21). NO is the most important diffusible mediator of endothelium-dependent vasodilation, with diverse paracrine effects on the intima and media layers (22). It is continuously synthesized in endothelial cells exposed to wall shear stress (23), where it opposes the actions of numerous vasoconstrictive agonists (24) and suppresses platelet adhesion and leukocyte infiltration (25). Diffusion of NO within the vessel wall also inhibits IH through cell cycle arrest of VSMCs and retardation of angiotensin II (AngII) –mediated VSMC migration from the media (22). In the absence of NO, other diffusible endothelium–derived factors, such as AngII and endothelin-1, lead to vasoconstriction, VSMC proliferation, and hypertrophic remodeling through their interaction with VSMC receptors (26). In agreement with this hypothesis, Skartsis et al. (27) have shown that neointimal cells of experimental AVFs are derived from activated medial VSMCs.
IH and AVF Outcomes
Several groups have evaluated the association of preexisting vascular IH with postoperative IH and AVF nonmaturation (Table 1). Two pilot studies reported a positive association between preexisting arterial or venous IH and AVF maturation failure (13,14). Kim et al. (14) observed preexisting arterial IH in 45 of 59 (76%) patients undergoing AVF creation. AVF failure was more frequent in patients with preexisting arterial IH. A very small pilot study (n=7) reported that greater preexisting venous IH was associated with AVF nonmaturation (13). In contrast, a much larger (n=129), prospective, single––center study found no association between preexisting venous IH and postoperative AVF stenosis (28). Finally, Tabbara et al. (10) assessed the contribution of preexisting venous IH to postoperative AVF pathology and clinical outcomes in a prospective study of 96 patients scheduled for two–stage transposition AVFs. Sequential venous samples were obtained: the first one from the native vein used for AVF creation and the second one several weeks later from the draining vein of the AVF at the time of AVF superficialization (Figure 1). Surprisingly, there was no correlation between the magnitude of preexisting and postoperative venous intimal thickness, suggesting that IH in AVF occurs by a different mechanism than that responsible for IH in the native vessel. In agreement with the work by Allon et al. (28), Tabbara et al. (10) found no correlation between preexisting IH and postoperative AVF stenosis. In summary, most of the published literature does not support a role for preexisting venous IH in the pathogenesis of postoperative IH or AVF maturation failure.
Medial Fibrosis
Pathobiology of Preexisting Medial Fibrosis
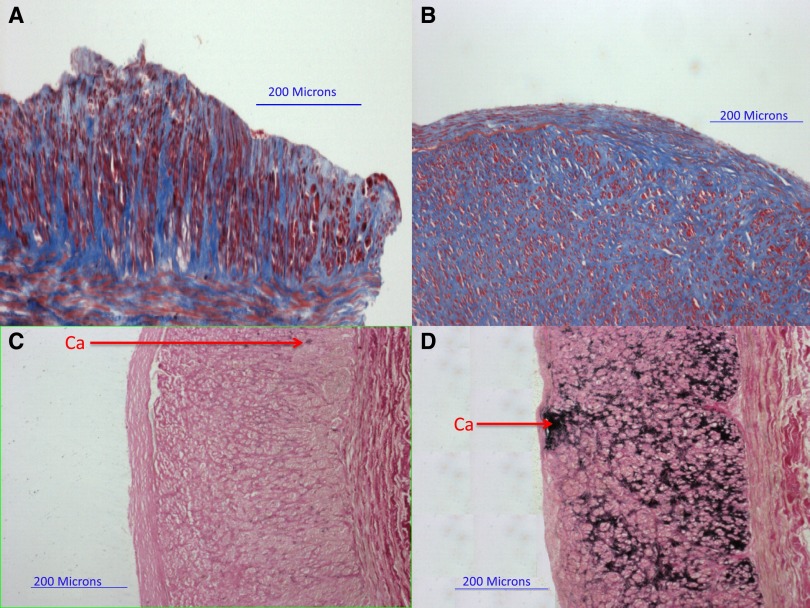
Arterial and venous fibrosis secondary to the accumulation of collagen types 1 and 3 in the medial layer is a common pathology in patients with CKD (Figure 2, A and B). It likely represents a response to elevated pulse pressures and high levels of AngII and TGF-β (29–31). Medial fibrosis occurs independently of vessel geometry, anatomic location, and susceptibility to atherosclerosis (32) but may reflect a poorly defined subclinical vascular remodeling process. Medial fibrosis and disorganized vascular muscle organization were highlighted after pathologic analysis of cephalic and basilic veins collected before AVF creation (33). Wali et al. (33) suggested a causative association between fibrosis and the irregular shapes, disrupted membranes, pseudopodia-like projections, and intracellular collagen–containing vacuoles of VSMCs populating the media of preaccess veins. This implies a contribution of vein fibrosis to the VSMC transformation toward the myofibroblastic and migratory phenotypes that are common in IH lesions and suggests a relationship between both preexisting vascular conditions.
Figure 2.
Pathologic abnormalities in arteries obtained during arteriovenous graft creation. (A and B) Masson's trichrome stain was used to quantify medial fibrosis (collagen stains blue). (C and D) Von Kossa stain was used to quantify arterial calcification (calcium [Ca] stains black). (A) Artery with mild medial fibrosis (49%). (B) Artery with severe medial fibrosis (86%). (C) Artery with mild microcalcification (0.4%). (D) Artery with severe microcalcification (47.1%). Reprinted from reference 55, with permission.
Whether medial fibrosis determines vascular access distention, maturation, and primary access patency remains uncertain, in part because of the absence of a standardized methodology for assessing vascular fibrosis and the parallel determination of the biomechanical properties of blood vessels before and after access creation. Interestingly, the forearm veins of patients with end stage renal failure are less elastic than those of healthy control individuals (34). In agreement, a small study of 17 patients found that greater venous distensibility was associated with better AVF maturation (35). Conversely, a pilot study found no association between peripheral arterial stiffness, as determined by pulse wave velocity, and subsequent AVF outcomes (21). The HFM study of approximately 600 patients found no significant association between venous plethysmography and the 6-week postoperative AVF diameter or blood flow (36), suggesting that preexisting distensibility does not modify flow–mediated vascular distention and compliance of the outflow vein. The configuration of collagen fibers within the arterial media of patients with CKD may affect AVF maturation (37). Finding a plausible mechanism to explain how collagen structure and fibrosis determine postanastomotic remodeling warrants additional investigation.
Medial Fibrosis and AVF Outcomes
Increased vascular stiffness caused by medial fibrosis has been proposed as a limiting factor for arterial dilation and AVF maturation in patients with CKD. A multivariate regression analysis showed an association between renal dysfunction and the magnitude of medial fibrosis in postmortem vascular fragments from 100 patients (32). Arterial medial fibrosis was associated with older age and diabetes in a pilot study of 50 patients with CKD undergoing AVF creation. However, there was no association between preexisting arterial medial fibrosis and AVF nonmaturation in this study (38). Finally, a larger prospective study by Allon and coworkers (37) (125 patients) unexpectedly found that greater preexisting arterial medial fibrosis was associated with greater 6-week AVF diameter and blood flow. In contrast, preexisting venous medial fibrosis was not associated with AVF maturation. This counterintuitive observation requires additional investigation to explain how an increase in arterial medial collagen might enhance AVF maturation. One potential mechanism might be that increased arterial medial fibrosis increases intra-arterial pressure and exposes the draining vein to a high pressure, thereby promoting its dilation. Alternatively, an unidentified third factor may promote arterial medial fibrosis and AVF maturation. There is no published information on the changes in vascular medial fibrosis after AVF creation, but these could be potentially assessed by studying paired vein samples obtained before and after AVF creation in patients undergoing a two-stage AVF.
Vascular Calcification
Pathobiology of Vascular Calcification
Vascular calcification is a common complication in CKD (Figure 2, C and D) and a known cause of arterial and venous stiffness (39). Vascular calcification occurs in both the intimal and medial layers, with intimal lesions being closely associated with atherosclerotic processes and medial calcification being associated with diabetes and CKD (40). Intimal calcification generates thrombosis because of plaque rupture, whereas medial lesions are associated with stiffness as a result of elastic fiber mineralization. Both intimal and medial calcifications have been observed in large arteries of patients with CKD (41). In contrast, medial calcification is highly prevalent in the peripheral (brachial and radial) arteries, particularly in patients with advanced CKD (41). Interestingly, preexisting calcification has been detected in all vascular layers of veins used for hemodialysis access creation from the endothelium to the adventitia (42).
Regardless of the vascular localization and the predominant role played by macrophages in the atherosclerotic intima, the underlying mechanism of vascular calcification is an imbalance between the cellular mediators that promote and inhibit mineralization (40). In addition to the dysregulation of calcium and phosphorus that is characteristic of CKD, the uremic milieu induces VSMC degeneration and upregulation of the osteogenic program as illustrated by higher expression of osteogenic phenotype markers and increased secretion of the promineralization factor BMP-2 from VSMCs (43,44). Patients with CKD on hemodialysis also have lower serum levels of fetuin-A, an osteogenic inhibitor produced by the liver (45), and a lower vascular wall expression of active matrix Gla protein, a vitamin K–dependent factor that suppresses mineralization (46). Increased duration of uremia correlates with calcification severity (17). In addition, hemodialysis accelerates vascular calcification, even in the pediatric CKD population, in which the atherosclerosis burden is low (47). It has been proposed that vascular calcification in this population is caused by an increment in VSMC apoptosis, which in turn, increases inflammation and the availability of apoptotic bodies that later become calcified (47). Both the presence and extent of arterial calcification are independent predictors of cardiovascular mortality in patients on hemodialysis (48).
Vascular Calcification and AVF Outcomes
The association of vascular calcification with AVF outcomes depends on the methods used for calcification diagnosis, with discrepant results reported with radiologic imaging and histologic analysis (Table 1). Calcification severity in patients on hemodialysis with working AVFs as determined by radiology correlated with worse AVF patency in a 5-year follow-up study (49). In contrast, using histologic analysis, preexisting microcalcification in arteries used for AVF creation did not predict postoperative stenosis, AVF maturation, or primary unassisted patency (38,50). Arterial macrocalcification, as determined by preoperative ultrasound, correlated well with arterial microcalcification and was also not associated with AVF nonmaturation (50). This study, however, excluded patients with severe concentric calcification, which is better detected by plain radiographs. Another study reported that arterial microcalcification was associated with 1-year primary unassisted patency in a retrospective cohort of 114 patients (51). The same group showed that microcalcification also predicts cardiovascular mortality in patients on hemodialysis (52). In conclusion, the available data are inconsistent with respect to the effect of vascular calcification on AVF outcomes, indicating that larger and multicenter studies are needed to finally answer this question.
Preexisting Vascular Pathology and AVG Outcomes
Although AVFs are the vascular access of choice for long-term hemodialysis, AVGs can be a life-sustaining solution for patients who are not good candidates for AVFs. Unlike AVFs, AVGs provide reliable blood flow rates shortly after their placement and do not require maturation. Because of the place of AVGs in therapy and the etiologic differences between AVF and AVG failure, it is important to understand the risk factors and mechanisms that lead to graft stenosis and thrombosis. For example, the prosthetic material of AVGs may incite a more prominent role of inflammatory cells, compliance mismatch, and platelet aggregation on access failure (53).
Recurrent stenosis in AVGs is mostly attributed to the development of occlusive IH at the graft-vein anastomosis (15,54). As expected, the types of cells isolated from graft IH lesions include not only the typical myofibroblasts characteristic of AVF lesions and a small proportion of contractile VSMCs (<10%) but also, an abundance of macrophages (54). Very few studies have evaluated the effect of preexisting vascular pathologies on AVG functional outcomes (Table 1). Allon et al. (55) reported in a prospective cohort of 76 patients that preexisting arterial and venous IHs were actually associated with a lower frequency of AVG interventions. Similarly, greater medial fibrosis or microcalcification of arteries was associated with a lower frequency of AVG interventions. In contrast, none of the above variables (arterial IH, venous IH, severe arterial medial fibrosis, and severe arterial calcification) were able to predict primary unassisted patency or the time to first access intervention (55). The lack of association of preexisting vascular pathology with primary unassisted AVG patency might indicate that the study was underpowered. Alternatively, the mechanisms leading to the first AVG stenosis may differ from those leading to restenosis after endovascular interventions. In the latter case, thicker intima layers and more pronounced fibrosis and calcification might restrict inflammatory cell infiltration and delay recurrent luminal occlusions.
Macrocalcification of pelvic arteries as determined by preoperative computed tomography scans or sonography was associated with immediate technical failure of thigh AVG placement because of the inability to complete the graft-artery anastomosis (56). Secondary thigh AVG survival (time to permanent failure) was also reduced in patients with moderate to severe preexisting arterial macrocalcification detected by duplex ultrasound (57). Additional studies are necessary to confirm these results in upper extremity vessels as well as the effect of preexisting IH, medial fibrosis, and microcalcification on AVG outcomes.
Conclusion and Future Directions
In summary, there is no compelling evidence that preexisting vascular IH, medial fibrosis, or microcalcification has any adverse effects on AVF nonmaturation or primary unassisted patency of AVFs and AVGs. Published investigations have shown no association between individual arterial or venous histopathologic findings and access outcomes in relatively small cohorts. The only two studies that simultaneously assessed multiple preexisting conditions on preaccess arteries failed to report any potential interaction between two or more of these vascular pathologies and vascular access outcomes (38,55). However, studies that integrate the potential effect of multiple venous pathologies are lacking. The available evidence, thus, highlights the necessity of future research in larger patient populations that involves a multifaceted evaluation of preexisting vascular pathologies and risk factors, including endothelial function.
Despite the lack of association between preexisting pathologies and access outcomes, improving preoperative vascular health in patients with CKD should start at earlier stages of the disease (well before the time of vascular access placement) to avoid a significant loss of vascular elasticity or compliance secondary to subclinical pathologies that may increase the likelihood for failure. Indeed, interventions to prevent vascular microcalcification, fibrosis, and IH can bring more benefits than detrimental effects to the overall cardiovascular system. There are many therapies that can be used to prevent those vascular preconditions that are associated with CKD. These include smoking cessation and better hyperlipidemia and diabetes management, which would also help decelerate preexisting atherosclerosis and intimal vascular calcification. We should start considering numerous drugs that are widely used to treat cardiovascular disease in patients with CKD (statins, angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, and some β-blockers) to maximize vascular function before access creation. Although observational studies have not found an association between the use of statins and vascular access outcomes (58), randomized, controlled trials are needed to definitively test their potential benefit. The β-blocker nebivolol improves vasodilation by increasing NO production (59) and may, therefore, be particularly beneficial in enhancing AVF maturation. In terms of vascular calcification, a tight control of calcium, phosphorus, and vitamin D levels should be maintained throughout the course of CKD. Finally, vitamin K antagonists should be avoided in patients with CKD whenever possible because of their propensity to aggravate vascular calcification (60). Maintaining an adequate BP control and lowering uremia levels will not only help with endothelial function but also, prevent the development or progression of IH and both intimal and medial vascular calcification. Of note, the serum urea level before AVF creation was associated with primary failure in a recent prospective study of >500 patients (61). A special emphasis should be made in the surgical training and referral pattern, because numerous studies revealed that surgical experience is a statistically significant predictor of success in vascular access placement (62).
Finally, it is imperative to apply powerful and new high–throughput technologies for probing the transcriptome, proteome, and metabolome in blood and preaccess vessels of patients with CKD. This comprehensive approach would allow the identification of biomarkers that predict different vascular access outcomes and may aid in patient–specific AVF planning. Using integrated multiomics approaches will undoubtedly increase our mechanistic understanding of the complex biologic systems underlying preexisting pathologies and vascular dysfunction. This strategy will eventually lead to the identification of novel therapeutic targets. More importantly, the application of systems biology approaches has the potential to open new avenues for personalized and precision medicine as well as the development of better tools to predict AVF outcomes.
Disclosures
M.A. is a consultant for CorMedix (Bedminster, NJ) and Gore (Flagstaff, AZ).
Acknowledgments
We thank Laisel Martinez for fruitful discussions and her critical revision of the manuscript.
National Institutes of Health grants R01DK098511 (to R.I.V.-P.) and R01DK085027 (to M.A.) supported this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Schinstock CA, Albright RC, Williams AW, Dillon JJ, Bergstralh EJ, Jenson BM, McCarthy JT, Nath KA: Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am Soc Nephrol 6: 1996–2002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, Kosa SD, Quinn RR, Moist LM: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee T, Barker J, Allon M: Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistula. J Am Soc Nephrol 18: 1936–1941, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Lee T, Woodle B, Wadehra D, Campos-Naciff B, Munda R: Balloon-assisted maturation (BAM) of the arteriovenous fistula: The good, the bad, and the ugly. Semin Nephrol 32: 558–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbin ML, Greene T, Cheung AK, Allon M, Berceli SA, Kaufman JS, Allen M, Imrey PB, Radeva MK, Shiu YT, Umphrey HR, Young CJ, Group FT: Arteriovenous fistula development in the first 6 weeks after creation. Radiology 279: 620–629, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shenoy S: Surgical anatomy of upper arm: What is needed for AVF planning. J Vasc Access 10: 223–232, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Lu DY, Chen EY, Wong DJ, Yamamoto K, Protack CD, Williams WT, Assi R, Hall MR, Sadaghianloo N, Dardik A: Vein graft adaptation and fistula maturation in the arterial environment. J Surg Res 188: 162–173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R: Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis 50: 782–790, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Tabbara M, Duque JC, Martinez L, Escobar LA, Wu W, Pan Y, Fernandez N, Velazquez OC, Jaimes EA, Salman LH, Vazquez-Padron RI: Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes [published online ahead of print March 22, 2016]. Am J Kidney Dis doi:10.1053/j.ajkd.2016.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feinfeld DA, Batista R, Mir R, Babich D: Changes in venous histology in chronic hemodialysis patients. Am J Kidney Dis 34: 702–705, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Wali MA, Eid RA, Dewan M, Al-Homrany MA: Pre-existing histopathological changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. Ann Thorac Cardiovasc Surg 12: 341–348, 2006 [PubMed] [Google Scholar]
- 13.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P: Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 26: 2264–2270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YO, Song HC, Yoon SA, Yang CW, Kim NI, Choi YJ, Lee EJ, Kim WY, Chang YS, Bang BK: Preexisting intimal hyperplasia of radial artery is associated with early failure of radiocephalic arteriovenous fistula in hemodialysis patients. Am J Kidney Dis 41: 422–428, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L: Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant 24: 2786–2791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monroy MA, Fang J, Li S, Ferrer L, Birkenbach MP, Lee IJ, Wang H, Yang XF, Choi ET: Chronic kidney disease alters vascular smooth muscle cell phenotype. Front Biosci (Landmark Ed) 20: 784–795, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibels LS, Alfrey AC, Huffer WE, Craswell PW, Anderson JT, Weil R 3rd: Arterial calcification and pathology in uremic patients undergoing dialysis. Am J Med 66: 790–796, 1979 [DOI] [PubMed] [Google Scholar]
- 18.Davies AH, Magee TR, Baird RN, Sheffield E, Horrocks M: Pre-bypass morphological changes in vein grafts. Eur J Vasc Surg 7: 642–647, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Baylis C: Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 294: F1–F9, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Wever R, Boer P, Hijmering M, Stroes E, Verhaar M, Kastelein J, Versluis K, Lagerwerf F, van Rijn H, Koomans H, Rabelink T: Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 19: 1168–1172, 1999 [DOI] [PubMed] [Google Scholar]
- 21.MacRae JM, Ahmed S, Hemmelgarn B, Sun Y, Martin BJ, Roifman I, Anderson T: Role of vascular function in predicting arteriovenous fistula outcomes: An observational pilot study. Can J Kidney Health Dis 2: 19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahanchi SS, Tsihlis ND, Kibbe MR: The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg 45[Suppl A]: A64–A73, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Andrews AM, Jaron D, Buerk DG, Kirby PL, Barbee KA: Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide 23: 335–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C: The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 10: 4–18, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Radomski MW, Palmer RM, Moncada S: Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2: 1057–1058, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Bourque SL, Davidge ST, Adams MA: The interaction between endothelin-1 and nitric oxide in the vasculature: New perspectives. Am J Physiol Regul Integr Comp Physiol 300: R1288–R1295, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Skartsis N, Manning E, Wei Y, Velazquez OC, Liu ZJ, Goldschmidt-Clermont PJ, Salman LH, Asif A, Vazquez-Padron RI: Origin of neointimal cells in arteriovenous fistulae: Bone marrow, artery, or the vein itself? Semin Dial 24: 242–248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Litovsky S: Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol 8: 1750–1755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson CP, Baugh R, Wilson CA, Burns J: Age related changes in the tunica media of the vertebral artery: Implications for the assessment of vessels injured by trauma. J Clin Pathol 54: 139–145, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J: TGF-beta signaling in vascular fibrosis. Cardiovasc Res 74: 196–206, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Ortega M, Ruperez M, Esteban V, Egido J: Molecular mechanisms of angiotensin II-induced vascular injury. Curr Hypertens Rep 5: 73–79, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Najjar SS, Cornish TC, Halushka MK: A comprehensive histopathological evaluation of vascular medial fibrosis: Insights into the pathophysiology of arterial stiffening. Atherosclerosis 208: 69–74, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wali MA, Eid RA, Al-Homrany MA: Smooth muscle changes in the cephalic vein of renal failure patients before use as an arteriovenous fistula (AVF). J Smooth Muscle Res 38: 75–85, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Kooman JP, Wijnen JA, Draaijer P, van Bortel LM, Gladziwa U, Peltenburg HG, Struyker-Boudier HA, van Hooff JP, Leunissen KM: Compliance and reactivity of the peripheral venous system in chronic intermittent hemodialysis. Kidney Int 41: 1041–1048, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Salman L, Vazquez-Padron RI, Castro H, Monrroy M, Abdelwahed Y, Rizvi A, Merrill D, Asif A: Measurement of vessel diameter during angioplasty: are we accurately performing this task? Semin Dial 27: E38–E41, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Allon M, Greene T, Dember LM, Vita JA, Cheung AK, Hamburg NM, Imrey PB, Kaufman JS, Robbin ML, Shiu YT, Terry CM, Umphrey HR, Feldman HI; Hemodialysis Fistula Maturation Study Group: Association between preoperative vascular function and postoperative arteriovenous fistula development [published online ahead of print May 9, 2016] J Am Soc Nephrol doi:ASN.2015020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiu YT, Litovsky SH, Cheung AK, Pike DB, Tey JCS, Zhang Y, Young CJ, Robbin M, Hoyt K, Allon M: Preoperative vascular medial fibrosis and arteriovenous fistula development. Clin J Am Soc Nephrol 2016, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58: 437–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moe SM, Chen NX: Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 19: 213–216, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J: Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant 31: 31–39, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Wang N, Yang J, Yu X, Hu J, Xing C, Ju X, Shen X, Qian J, Zhao X, Wang X: Radial artery calcification in end-stage renal disease patients is associated with deposition of osteopontin and diminished expression of alpha-smooth muscle actin. Nephrology (Carlton) 13: 367–375, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Lee T, Safdar N, Mistry MJ, Wang Y, Chauhan V, Campos B, Munda R, Cornea V, Roy-Chaudhury P: Preexisting venous calcification prior to dialysis vascular access surgery. Semin Dial 25: 592–595, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen NX, O’Neill KD, Duan D, Moe SM: Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int 62: 1724–1731, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX: Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int 63: 1003–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W: The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112: 357–366, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Proudfoot D, Shanahan CM: Molecular mechanisms mediating vascular calcification: Role of matrix Gla protein. Nephrology (Carlton) 11: 455–461, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM: Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118: 1748–1757, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Jankovic A, Damjanovic T, Djuric Z, Marinkovic J, Schlieper G, Tosic-Dragovic J, Djuric P, Popovic J, Floege J, Dimkovic N: Impact of vascular calcifications on arteriovenous fistula survival in hemodialysis patients: A five-year follow-up. Nephron 129: 247–252, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Allon M, Robbin ML, Umphrey HR, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Barker-Finkel J, Litovsky S: Preoperative arterial microcalcification and clinical outcomes of arteriovenous fistulas for hemodialysis. Am J Kidney Dis 66: 84–90, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi SJ, Yoon HE, Kim YS, Yoon SA, Yang CW, Kim YS, Park SC, Kim YO: Pre-existing arterial micro-calcification predicts primary unassisted arteriovenous fistula failure in incident hemodialysis patients. Semin Dial 28: 665–669, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Yun YS, Choi SJ, Lee JY, Kim YS, Yoon SA, Park SC, Shin OR, Jang EJ, Kim YO: Impact of arterial microcalcification of the vascular access on cardiovascular mortality in hemodialysis patients. Hemodial Int 18: 54–61, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Li L, Terry CM, Shiu YT, Cheung AK: Neointimal hyperplasia associated with synthetic hemodialysis grafts. Kidney Int 74: 1247–1261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC: Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int 59: 2325–2334, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Correlation of pre-existing vascular pathology with arteriovenous graft outcomes in hemodialysis patients. Am J Kidney Dis 62: 1122–1129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lockhart ME, Robbin ML, McNamara MM, Allon M: Association of pelvic arterial calcification with arteriovenous thigh graft failure in haemodialysis patients. Nephrol Dial Transplant 19: 2564–2569, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Little MD, Allon M, McNamara MM, Ong S, Lockhart ME, Young CJ, Robbin ML: Risk evaluation of immediate surgical failure during thigh hemodialysis graft placement by sonographic screening. J Ultrasound Med 34: 1613–1619, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Pisoni R, Barker-Finkel J, Allo M: Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol 5: 1447–1450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss R: Nebivolol: A novel beta-blocker with nitric oxide-induced vasodilatation. Vasc Health Risk Manag 2: 303–308, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett WM: Should dialysis patients ever receive warfarin and for what reasons? Clin J Am Soc Nephrol 1: 1357–1359, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Aitken E, Jackson A, Kong C, Coats P, Kingsmore D: Renal function, uraemia and early arteriovenous fistula failure. BMC Nephrol 15: 179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]