Table 5.
c-Kit inhibitors classification based on their targets, chemical and structure formulae, and diseases they are tested on
| Name | Targets | IC50a (nM) | Structure | Formula | Molecular weight (g/mol) | Chemical name | FDA-approved inhibitor | Clinical trial information testing on |
|---|---|---|---|---|---|---|---|---|
| Amuvatinib (MP-470) | c-Kit, PDGFRα, Flt3 | 10, 40, 81 |
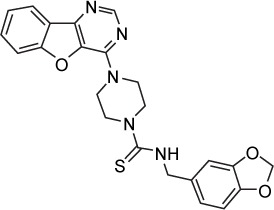
|
C23H21N5O3S | 447.51 | Not mentioned | Approved by the FDA for CML, GISTs and a number of other malignancies | Lymphoma, unspecified adult solid tumor, solid tumors, malignant disease, small-cell lung carcinoma |
| Axitinib | VEGFR1, VEGFR2, VEGFR3, PDGFRβ, c-Kit | 0.1, 0.2, 0.1–0.3, 1.6, 1.7 |
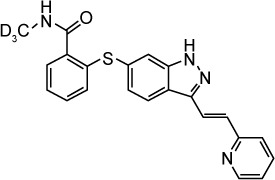
|
C22H18N4OS | 386.47 | Approved by the FDA | Advanced renal cell carcinoma, renal cell carcinoma, nonclear cell, temsirolimus-resistant renal cell carcinoma, pheochromocytoma, paraganglioma, advanced solid tumors | |
| Cabozantinib (XL184, BMS-907351) | VEGFR2, c-Met, Ret, Kit, Flt-1/3/4, Tie2, AXL | 0.035, 1.3, 4, 4.6, 12/11.3/6, 14.3, 7 |
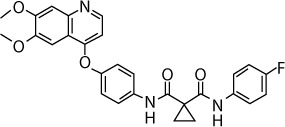
|
C28H24FN3O5 | 501.51 | Approved by the FDA for renal cell carcinoma | Medullary thyroid cancer, prostate cancer, castration-resistant prostate cancer, prostatic neoplasms, colorectal cancer, uterine sarcoma, and prostate cancer | |
| Dasatinib | Abl, Src, c-Kit | 1, 0.8, 79 |
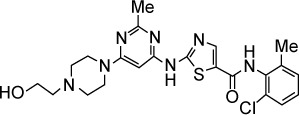
|
C22H26ClN7O2S | 488.01 | Approved by the FDA for Ph+ CML | AML, breast cancer, recurrent childhood brain tumor, lung cancer/NSCLC, chronic myeloid leukemia | |
| Dovitinib (TKI-258, CHIR-258) | FLT3, c-Kit, FGFR1/3, VEGFR1-4, InsR, EGFR, c-Met, EphA2, Tie2, IGF-1R, and HER2 | 1/2, 8–13 |
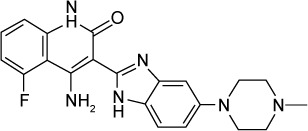
|
C21H21FN6O | 392.43 | Propanoic acid, 2-hydroxy-, compound with 4-amino-5-fluoro-3-[6-(4-methyl-1-piperazinyl)- 1H-benzimidazol-2-yl]-2(1H)-quinolinone |
Notes: Data from Selleckchem.com, http://www.selleckchem.com/c-Kit.html####.
The IC50 is a measure of the effectiveness of a substance in inhibiting a specific biological or biochemical function.
Abbreviations: IC50, half-maximal inhibitory concentration; FDA, Food and Drug Administration; CML, chronic myelogenous leukemia; GIST, gastrointestinal stromal tumor; AML, acute myeloid leukemia; NSCLC, non-small-cell lung cancer.
