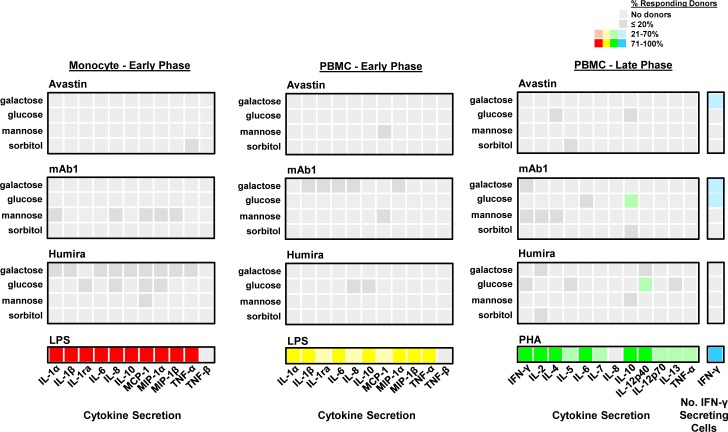
Fig 5. Chemical Modification by glycation does not enhance the response of PBMC in the IVCIA assay.
Three representative mAbs (Avastin, mAb1, and Humira), that were treated by glycation with different sugars (galactose, glucose and mannose, and a non-glycating sugar, sorbitol), were tested in the IVCIA assay at the early (20 h) (n = 11 donors for most samples) and late (7 day) phases (n = 6 donors). Avastin, mAb1, and Humira have low, medium, and high rates of clinical or predicted immunogenicity, respectively. Heatmaps depict the percentage of donors that responded to the glycated mAbs above the original forms of each molecule (mAb before glycation treatment) and were at least two fold above the background, to highlight the differential response that might be due to glycated mAbs. The percentage of donors with increased secretion of signature cytokines in adherent monocytes at the early phase (red), and in PBMC at the early (yellow) and late (green) phases is highlighted. The percentage of donors with an increase in the number of IFN-γ secreting cells is shown on the far right (blue). The grey boxes show low level responses that were observed in less than or equal to 20% of donors, in contrast to colored boxes (red, yellow, green and blue) that show responses in a greater number of donors (40–100%).