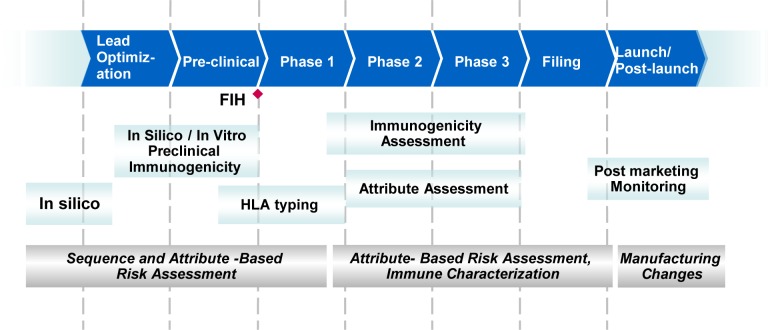
Fig 7. Stages of biotherapeutic development where the IVCIA assay can be used for risk assessment related to attributes.
In silico or algorithm based assessments rank order and identify lead candidates based on the least sequence based risk. In vitro assessments identify non-sequence attributes and any immune mediated target based risk at preclinical stage prior to first in human (FIH). Pharmacogenomic assessments for HLA can be introduced in long-term clinical studies (Phase 1b/2) to evaluate associations of HLA with clinical immunogenicity. Immunogenicity assessments measured in serum as ADA span the breadth of clinical trials and disease indications (FIH to Launch/post launch). In vitro assays also provide attribute related risk assessment of manufactured lots, lot-to-lot comparability, and risk post packaging due to attributes related to storage, shipping, handling, and device-related leachates.