Abstract
Decidual natural killer (dNK) cells have been shown to both promote and inhibit trophoblast behavior important for decidual remodeling in pregnancy and have a distinct phenotype compared to peripheral blood NK cells. We investigated whether different levels of oxygen tension, mimicking the physiological conditions of the decidua in early pregnancy, altered cell surface receptor expression and activity of dNK cells and their interactions with trophoblast. dNK cells were isolated from terminated first-trimester pregnancies and cultured in oxygen tensions of 3%, 10%, and 21% for 24 h. Cell surface receptor expression was examined by flow cytometry, and the effects of secreted factors in conditioned medium (CM) on the trophoblast cell line SGHPL-4 were assessed in vitro. SGHPL-4 cells treated with dNK cell CM incubated in oxygen tensions of 10% were significantly more invasive (P < 0.05) and formed endothelial-like networks to a greater extent (P < 0.05) than SGHPL-4 cells treated with dNK cell CM incubated in oxygen tensions of 3% or 21%. After 24 h, a lower percentage of dNK cells expressed CD56 at 21% oxygen (P < 0.05), and an increased percentage of dNK cells expressed NKG2D at 10% oxygen (P < 0.05) compared to other oxygen tensions, with large patient variation. This study demonstrates dNK cell phenotype and secreted factors are modulated by oxygen tension, which induces changes in trophoblast invasion and endovascular-like differentiation. Alterations in dNK cell surface receptor expression and secreted factors at different oxygen tensions may represent regulation of function within the decidua during the first trimester of pregnancy.
Keywords: decidual natural killer cell, NKG2D, oxygen, trophoblast
INTRODUCTION
A healthy pregnancy is dependent on interactions between fetal placental trophoblast cells and maternal cells of the uterine wall (decidua). During the first 12 wk of pregnancy, extravillous trophoblasts (EVT) invade the decidua and remodel uterine spiral arteries from high-resistance, low-flow vessels to high-flow vessels to supply nutrients to the growing fetus. Failure to adequately remodel these vessels has been associated with pregnancy disorders including pre-eclampsia and miscarriage [1]. Decidual natural killer (dNK) cells are the most abundant leukocyte in the decidua [2] and are distinct from peripheral blood (pb)NK cells due to their low cytotoxicity and high cytokine-secreting activity [3]. This unique phenotype may aid immune tolerance of the semiallogeneic fetus and play a key role in regulation of the trophoblast-dependent process of spiral artery remodeling.
Decidual NK cells have been shown to both promote and inhibit trophoblast behavior important for the spiral artery remodeling such as motility, invasion, and chemotaxis [4–6]. The phenotype of dNK cells, which is distinct compared to that of pbNK cells, is marked predominantly by CD56bright, a lack of CD16 expression, and higher expression of certain cell surface receptors including LILRB1, KIRs, NKp46, and NKp30 [7, 8]. The reasons for differences between dNK cells and cytotoxic pbNK cells are still unknown; however, the decidual microenvironment may play a key role in modulation of the dNK phenotype.
During the first trimester of pregnancy, the fetus and placenta develop in a low-oxygen environment, in which a steep rise in oxygen tension is correlated with increasing gestational age between 10 and 12 wk, from approximately 20 to 60 mm Hg (2%–10% oxygen) [9]. Although this range is referred to as hypoxia, it is in fact normoxic for the implantation site [10]. Because the decidua is a vascularized tissue, it is more oxygenated than the placenta, but similarly to the placenta, oxygen levels in the decidua rise between 10 and 12 wk of gestation from approximately 50 to 70 mm Hg (approximately 8%–12% oxygen [9]), although other investigators have estimated higher oxygen pressures, affecting cells adjacent to the spiral arteries at 90–100 mm Hg (approximately 20% oxygen) [11]. However, in the part of the decidua closest to the intervillous space, oxygen levels are affected by spiral artery plugging by trophoblast, and oxygen is thought to be reduced compared to the rest of the decidua, forming a steep decreasing oxygen gradient from the arteries to the intervillous space [10]. This decreasing oxygen gradient effects trophoblast behavior, and oxygen modulation has been shown to be important for differentiation of proliferating cytotrophoblasts in cell columns into invasive EVT [12].
The oxygen gradient may also have an impact on dNK cells surrounding plugged spiral arteries and may influence their interactions with trophoblast. This has been indicated by the induction of pbNK cells into a dNK-like phenotype with a combination of factors mimicking the decidual environment, including hypoxia and TGF-β, and the evidence that dNK cells lose decidual features and gain cytotoxicity upon long-term culture [13]. In other examples of low-oxygen environments such as the hypoxic tumor microenvironment, NK cells display decreased cytotoxicity [14, 15] and modulation of cell surface receptors [15]. In this study we aimed to investigate the effects that oxygen tensions at approximately 2%, 8%, and 20%, mimicking physiological conditions of different areas of the decidua, had on the phenotype and activity of dNK cells.
MATERIALS AND METHODS
Tissue Collection
Decidual tissue was collected from women attending a clinic for termination of pregnancy in the first trimester, as previously described [16], at the Fetal Medicine Unit, St. George's Hospital. Ethics committee approval and full written consent were obtained. Inclusion criteria were singleton pregnancy, gestational age of 6–13 wk, normal fetal anatomy, and nuchal translucency thickness with no known maternal medical condition or history of recurrent miscarriage.
Decidual Natural Killer Cell Isolation
Decidual NK cells were isolated as previously described [6]. Briefly, decidual tissue was minced and digested in serum-free M199 medium containing 2 mg/ml collagenase and 0.1 mg/ml DNase overnight with constant agitation at room temperature. Resultant tissue digest was passed sequentially through 100-μm and 70-μm filters and layered onto Ficoll-Paque (GE Healthcare Life Sciences, Buckinghamshire, U.K.). The buffy layer was collected, and cells were resuspended in 10 ml of dNK cell culture medium (phenol red-free RPMI 1640 medium supplemented with 10% [v/v] fetal bovine serum [FBS] containing 2 mmol/L L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin) and plated on cell culture dishes in a 37°C incubator for 15 min. Nonadherent cells containing the dNK cell fraction were then purified by negative selection using a MagCellect human NK cell isolation kit (R&D Systems, Abingdon, U.K.) according to the manufacturer's instructions. Purity of cells as measured by CD56-positivity was, on average, 95.7 ± 0.92%, and viability immediately upon isolation was 96.5 ± 0.38% (n = 33), as assessed by fixable viability dye (eBioscience, Hatfield, U.K.).
Cell Culture
SGHPL-4 cells are a well-characterized extravillous trophoblast cell line derived from primary human first-trimester EVT [17]. SGHPL-4 cells were cultured in Hams F10 medium (Sigma-Aldrich, Dorset, U.K.) supplemented with 10% (v/v) FBS containing L-glutamine (2 mmol/L), penicillin (100IU/ml), and streptomycin (100 μg/ml; Invitrogen, Paisley, U.K.). All cells were incubated with 95% air and 5% CO2 at 37°C in a humidified incubator. Decidual NK cells were cultured at 1 × 106 cells/ml in RPMI 1640 medium (Invitrogen) with 10% (v/v) FBS containing 2.5 μg/ml amphotericin B (Sigma-Aldrich), 2 mM L-glutamine, 50 μg/ml penicillin, and 50 μg/ml streptomycin (Invitrogen), 50 ng/ml stem cell factor and 5 ng/ml interleukin-15 (IL-15; Peprotech, London, U.K.) at 37°C.
Oxygen Culture
Decidual NK cells at a concentration of 1 × 106 cells/ml were cultured in flasks placed inside sealed blood bags (Baldwin Medical Supplies, Knoxville, VIC, Australia) flushed with 1% oxygen (O2) with 5% CO2 in N2, 8% O2 with 5% CO2, or 20% O2 with 5% CO2 in N2 as previously described [18]. Over a 24-h period, this gave average O2 concentrations of 2.7% ± 0.35, 9.8 ± 0.21% and 20.9 ± 0.07%, respectively, as measured with a Pac 7000 model oxygen monitor (Draeger Healthcare, Germany [see Supplemental Fig. S1; available online at www.biolreprod.org] n = 3). This has been rounded up to 3%, 10%, and 21% in this article. Viability of dNK cells did not differ between treatments as assessed by fixable viability dye (eBioscience;). Decidual NK cell-conditioned medium (CM) was collected after 24 h, and to ensure any downstream applications were not altered by O2 concentration, medium was concentrated 10× by using a 6-ml Vivaspin column (Sartorius Stedim, Surrey, U.K.) and rediluted with fresh medium for use. dNK cells were used for flow cytometry.
Flow Cytometry
Freshly isolated and O2-cultured dNK cells were resuspended in 1 ml of phosphate-buffered saline (PBS) and stained with fixable viability dye Efluor 780 according to the manufacturer's instructions (eBioscience). Cells were then washed in Fluorescence-activated cell sorting (FACS) buffer (PBS with 0.5% [w/v] bovine serum albumin, 0.05% [w/v] sodium azide) and blocked in 1 μg/ml human immunoglobulin G (IgG). A sample of 2 × 105 cells were resuspended in 100 μl of FACS buffer, and cells were labeled using the following antibodies: mouse anti-human CD56-Alexa Fluor 488, mouse anti-human CD158b (KIR2DL2/S2)-PE, mouse anti-human NKG2D-APC, mouse anti-human NKp46-PE, mouse anti-human NKp30-PE (BD Pharmingen), mouse anti-human CD3-PerCP, mouse anti-human KIR2DL1/S1-APC (eBioscience), and mouse anti-human LILRB-1/ILT2/CD85j-APC. The following isotype controls were used: mouse IgG1 κ-Alexa Fluor 488, mouse IgG2b κ-PE, mouse IgG2a κ-APC, mouse IgG2b κ-APC, mouse IgG1 κ-APC, mouse IgG1 κ-PE (eBioscience), and mouse IgG1 κ-PerCP (BD Pharmingen).
Invasion Assay
The effect of dNK cell CM from cells cultured at different O2 concentrations on trophoblast invasion was measured as previously described [6]. Briefly, SGHPL-4 cells were cultured in three-dimensional (3D) spheroids and resuspended in 100 μl of fibrin gel (2.5 mg/ml fibrin, 100 U/ml aprotinin, 1.25 U/ml thrombin) in separate wells of a 96-well plate. Decidual NK cell CM from cells cultured at different O2 concentrations, as described, was added to each well with a control consisting of dNK culture medium with 50 ng/ml stem cell factor and 5 ng/ml IL-15. Spheroids were visualized after 24-h incubation, using an IX81 model inverted microscope (Olympus, Tokyo, Japan), and images were captured using a C4742-95 model digital camera (Hamamatsu, Tokyo, Japan). Invasion was measured as the average number and length of all invasive processes from each spheroid, using ImageJ version 1.47d software (U.S. National Institutes of Health, Bethesda, MD).
Chemotaxis Assay
SGHPL-4 cell chemotaxis toward dNK cell CM from cells cultured at different O2 concentrations was measured as previously described [6] using the μ-Slide chemotaxis 2D assay (Ibidi, Martinsried, Germany) according to the manufacturer's instructions. Analysis of chemotaxis and motility was carried out by time-lapse microscopy using a IX81 model inverted microscope with motorized stage and cooled charge-coupled device camera (Olympus) and enclosed in a heated, humidified chamber at 37°C with 5% CO2 in air. Images were taken every 15 min for 24 h, and time-lapse sequences were analyzed using ImageJ version 1.43u software (NIH) with the manual tracking and chemotaxis tool (version 1.01) plug-ins.
Trophoblast Network-Formation Assay
The ability of SGHPL-4 cells to form endothelial-like “tube” networks on Matrigel (BD Pharmingen, Oxford, U.K.) in the presence of dNK cell CM from cells cultured at different O2 concentrations was assessed using a μ-Slide angiogenesis assay according to the manufacturer's instructions and assessed by total branch length at 8 h, which took into account the length and number of branches formed.
Statistics
Data were analyzed where appropriate with Friedmann nonparametric ANOVA with Dunn multiple analysis post hoc test, using Prism version 6.01 software (GraphPad, La Jolla, CA).
RESULTS
Differences in Oxygen Tension Do Not Alter dNK Cell CM Effect on Trophoblast Motility or Chemotaxis
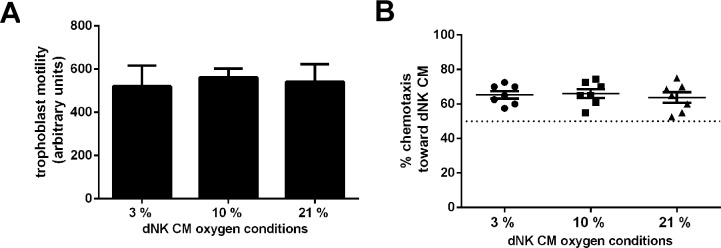
To determine whether different oxygen tensions alter the ability of dNK cells to induce trophoblast motility and chemotaxis as previously shown [6, 19], dNK cells were cultured in 3%, 10%, and 21% O2 for 24 h, and medium was collected. Motility of the EVT-like cell line SGHPL-4 was not altered when incubated with CM collected from dNK cultured under different oxygen conditions (Fig. 1A, 3% O2, 520.8 ± 38.8; 10% O2, 562 ± 39.9; 21% O2, 541.8 ± 33.3 arbitrary units). Chemotaxis of SGHPL-4 was assessed as the percentage of cells moving toward the stimulus of dNK cell CM. SGHPL-4 cells migrating toward dNK cell CM collected from all O2 conditions was demonstrated by figures of greater than 50% chemotaxis (which represents nondirected migration). However these were not significantly different from each other (Fig. 1B, 3% O2, 65.4 ± 2.1%; 10% O2, 66.1 ± 2.1%; 21% O2, 63.7 ± 2.1%).
FIG. 1.
Culture of dNK cells under different oxygen conditions does not alter their effect on SGHPL-4 cell motility and chemotaxis. A) Motility of SGHPL-4 cells incubated with dNK cell CM and collected under different oxygen conditions was not significantly different (arbitrary units, as assessed by time-lapse photomicroscopy). Motility assay data shown are means ± SEM (n = 7 repeats of the experiment with dNK from 7 individual patients). B) SGHPL-4 cells were cultured in chemotaxis chambers to analyze chemotactic capacity of dNK cell CM collected under different oxygen conditions. Nondirected movement gives expected chemotaxis of 50% of cells (dotted line). There were no differences in SGHPL-4 cell chemotaxis when treated with dNK cell CM collected under different oxygen conditions. Data shown are mean ± SEM (n = 7 repeats of the experiment with dNK from 7 individual patients).
Decidual NK Cell CM Cultured at 10% O2 Increases Trophoblast Invasion
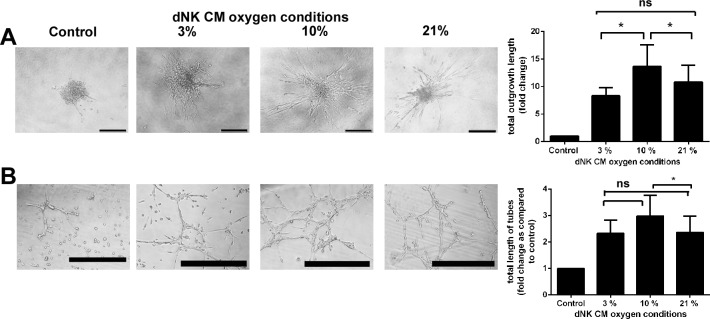
We previously demonstrated that dNK cell CM was able to induce SGHPL-4 invasion [6]. We aimed to determine whether culturing dNK under different oxygen tensions altered the induction of SGHPL-4 invasion. Decidual NK cell CM cultured under all O2 tensions induced SGHPL-4 invasion compared to control medium, and CM collected from dNK cultured in 10% O2 induced significantly more invasion than CM collected from dNK cultured in 3% O2 or 21% O2 (Fig. 2A, P < 0.05, 8.3 ± 1.5-fold change over control in 3% O2; 13.6 ± 4.7-fold change in 10% O2; and 10.8 ± 3.9-fold change in 21% O2).
FIG. 2.
Culture of dNK cells under different oxygen conditions alters the induction of trophoblast invasion and endothelial-like network formation. A) SGHPL-4 cells were cultured to form spheroids, as shown, and embedded in fibrin gel. Length and number of invasive processes were measured after incubation with dNK cell CM to determine total outgrowth length, which was increased in response to dNK cell CM collected at 10% O2 (*P < 0.05, n = 10 repeats of the experiment with dNK from 10 individual patients, bar = 100 μm). B) SGHPL-4 cells were cultured on Matrigel to induce endothelial-like network formation, which was assessed by counting the number and length of branches. Total length was increased in response to dNK cell CM cultured at 10% O2 compared to those at 2.6% and 21% O2 (*P < 0.05, n = 4 repeats of the experiment with dNK from 4 individual patients, bar = 500 μm). Data are means ± SEM; ns = not significant.
Decidual NK Cell CM Cultured at 10% O2 Increases Trophoblast Network Formation
Differentiation of EVT into endovascular endothelial-like cells lining the spiral arteries is the final step in trophoblast invasion into the decidua [20]. Decidual NK cells have been previously demonstrated to influence this [21]; therefore, we examined whether this was regulated by the oxygen conditions under which dNK cells were cultured. SGHPL-4 network formation was enhanced by dNK cell CM cultured at all O2 concentrations, and this was significantly increased after 8 h by dNK cell CM cultured at 10% compared to that at 21% O2 (Fig. 2B, P < 0.05; 2.3 ± 0.5-fold change in total branch length compared to control in 3% O2; 2.9 ± 0.7-fold change in 10% O2; and 2.3 ± 0.6-fold change in 21% O2).
Differences in Oxygen Tension Alter dNK Cell Surface Receptor Phenotype
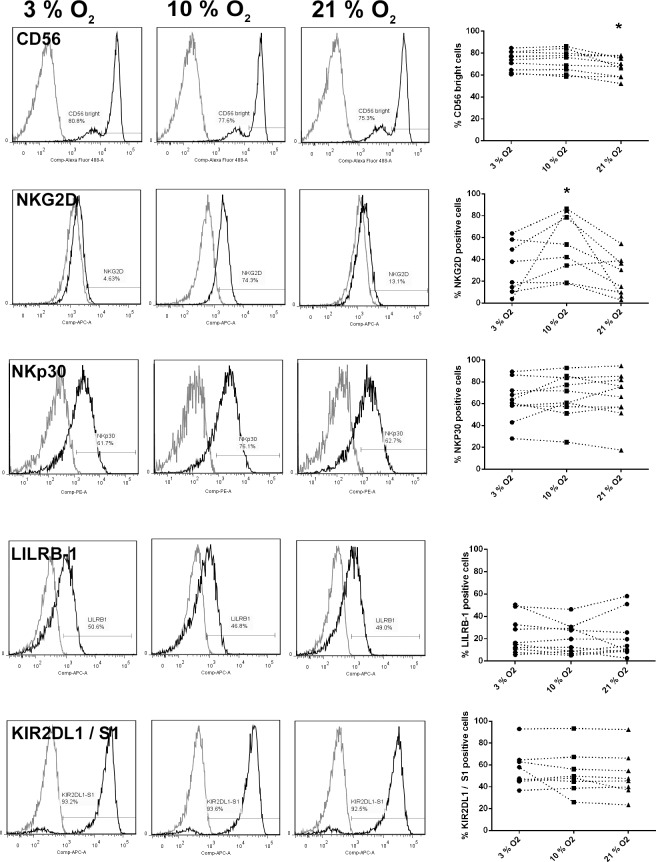
To examine whether differing O2 conditions altered the phenotype of dNK cells, flow cytometry was performed after 24 h of culture of dNK cells in 3%, 10%, and 21% O2 and expression levels of CD56, NKG2D, NKp30, LILRB1, and KIR2DL1 were assessed, as signaling via these receptors has previously been demonstrated to stimulate key functions of dNK cells [22–24]. No differences in cell viability were observed over the time period between different O2 concentrations (data not shown). Decidual NK cells are CD56bright, and this was found to significantly decrease after culture in 21% O2 compared to 3% or 10% O2 (Fig. 3, P < 0.05). Expression of the activating receptor NKG2D was found to be significantly increased at 10% O2 (Fig. 3, P < 0.05). Surface expression levels of NKp30, LILRB1, and KIR2DL1/S1 were not affected (Fig. 3).
FIG. 3.
Surface expression of receptors on dNK cells cultured for 24 h in 3%, 10%, or 21% O2. Data indicate representative phenotypes and pooled data for dNK cells after 24 h in culture, assessed by flow cytometry for the cell surface receptors listed. Gray line = IgG control, and darker line = test antibody. Data points show individual patient samples (n = 8).
DISCUSSION
In the decidua, resident NK cells are exposed to oxygen gradients in the first trimester of pregnancy which are temporally and regionally altered depending on gestational stage and extent of spiral artery plugging by trophoblast. Different oxygen tensions have been demonstrated to affect cells at the maternal–fetal interface; for example, a low-oxygen environment alters differentiation of EVT [25]. Our evidence suggests that the expression of secreted factors by dNK cells and their cell surface receptor phenotype may differ depending on the oxygen tension, altering their interactions with EVT.
In this study, the chemotaxis and motility of SGHPL-4 cells induced by dNK cell CM were not altered by the oxygen tension in which dNK cells were cultured. However, invasion and differentiation into networks were increased when incubated with CM of dNK cells exposed to 10% oxygen. Decidual NK cells secrete a number of angiogenic factors and cytokines, including vascular endothelial growth factor (VEGF), leukemia inhibitory factor (LIF), and several interleukins [26–28]. The relative levels of these factors will alter the effect that the dNK cell has on trophoblast. For example, some cytokines expressed by dNK cells are critical to trophoblast chemotaxis, including IL-8 and CXCL10 [4], whereas HGF has been demonstrated to be more important for trophoblast motility [19]. The invasion of trophoblast will also depend on their production of matrix metalloproteases (MMPs), which can be induced by dNK cell-secreted factors including IL-6 and IL-8 [29, 30]. Differentiation of EVT into endothelial-like cells involves the alteration of specific adhesion molecules and can be induced by the cytokine IL-8 [30]. Therefore, it would be relevant in larger future studies to determine how the factors secreted by dNK are altered under different oxygen conditions, and if absolute levels of secreted factors differ or if different cytokines and factors are secreted under different conditions. Additionally, a number of these factors including VEGF and pro-inflammatory cytokines are under the control of hypoxia inducible factor-1α (HIF-1α) in other innate immune cells [15, 31], and activation of this may impact on the levels of many of these secreted factors [32].
Decidual NK cells cultured at 20% oxygen were less able to promote SGHPL-4 cell invasion. This may reflect the fact that as trophoblast reach the higher oxygen conditions of the spiral arteries and the endpoint of their migration, promotion of invasion is less crucial, in a mechanism similar to the differentiation of the EVT seen at this oxygen tension [11]. However, this would also predict a greater extent of SGHPL-4 network formation induced by dNK cultured at 20% oxygen, when in fact we saw an increase at 10% oxygen in both invasion and network formation compared to both 3% and 21%. Although oxygen tension within the decidua alters with gestational age, we saw no differences in response of dNK cells isolated from a range of 6–13 wk in their responses to oxygen tension (data not shown). However, the effect of 10% oxygen on the ability of dNK cells to increase SGHPL-4 invasion and network formation may reflect subtle differences within the decidua depending on gestational age and position relative to plugged spiral arteries, as this may be the optimal oxygen conditions for dNK cells. We may also see additional effects if trophoblast were also cultured under the same oxygen conditions, which would be an interesting aspect to investigate further.
In addition to altered secretion of dNK factors, we determined there was a significantly lower percentage of CD56bright dNK cells after 24-h culture in 21% oxygen. This was not due to increased cell death under these conditions, as the proportion of live cells remained the same in each condition. This finding is in line with other studies that showed that low-oxygen tensions of 2% induce CD56bright expression [13]. A loss of CD56 expression could indicate a gain of cytotoxicity, as pbNK cells, which are CD56dim, have been demonstrated to have increased cytotoxic capability at 20% oxygen [33] or could also imply a loss of the CD56bright dNK phenotype. Culture of pbNK cells in hypoxia for periods of 7 days showed altered KIR and CD9 expression [13]; however, in our studies, expression of no other cell surface receptor changed, with the exception of NKG2D. This may reflect the relatively short time of 24 hours they were kept in culture to ensure that the cells remained viable.
Expression of NKG2D on dNK cells was found to be significantly increased after 24 h-culture in 10% oxygen. NKG2D is an activating receptor which has previously been shown on dNK cells to be important in the secretion of cytokines including IL-8 and IFN-γ [23], as well as the gain of cytotoxicity during viral infection [34]. NKG2D expression has been demonstrated to increase with gestational age in the first trimester of pregnancy [35], and this study indicates this may be related to oxygen levels. The outcome of increased NKG2D expression on dNK cells in 10% oxygen is unclear, but it may lead to increased signaling through this receptor, which could result in an altered cytokine secretion profile. Indeed, different dNK cytokine profiles have been demonstrated at different gestational ages [36]. Alternatively, increased NKG2D expression could lead to increased cytotoxicity [37]. This receptor has been previously implicated in the pathology of recurrent miscarriage, and as oxidative stress also upregulates the ligand of NKG2D [38], the importance of oxygen in the cell surface expression of this receptor should be further investigated. It may be interesting to investigate in the context of hypoxia-oxygenation, as this has been associated with pregnancy disorders [39].
Many aspects of the decidual environment will shape receptor expression and function of dNK cells, for example, decidual stromal cell ligand presentation [34] and secreted factors including TGF-β [13], IL-15 [40], and prolactin [41]. This study demonstrates that oxygen is also important in modulation of dNK cell surface receptor repertoire as well as the factors they secrete. Alterations in dNK cell surface receptor expression and secreted factors at different oxygen tensions may represent an additional level of local and gestational age-dependent regulation of function within the decidua during the first trimester of pregnancy.
ACKNOWLEDGMENT
The authors acknowledge Prof. Baskaran Thilaganathan and the staff of the Fetal Medicine Unit at St. George's Hospital for their assistance with sample collection.
Footnotes
This study was supported by the Wellcome Trust, project reference 091550.
REFERENCES
- Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140:803–813. doi: 10.1530/REP-10-0294. [DOI] [PubMed] [Google Scholar]
- King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update. 1998;4:480–485. doi: 10.1093/humupd/4.5.480. [DOI] [PubMed] [Google Scholar]
- Santoni A, Zingoni A, Cerboni C, Gismondi A. Natural killer (NK) cells from killers to regulators: distinct features between peripheral blood and decidual NK cells. Am J Reprod Immunol. 2007;58:280–288. doi: 10.1111/j.1600-0897.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–8530. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- Wallace AE, Host AJ, Whitley GS, Cartwright JE. Decidual natural killer cell interactions with trophoblasts are impaired in pregnancies at increased risk of preeclampsia. Am J Pathol. 2013;183:1853–1861. doi: 10.1016/j.ajpath.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Costa H, Tabiasco J, Berrebi A, Parant O, Aguerre-Girr M, Piccinni MP, Le Bouteiller P. Effector functions of human decidual NK cells in healthy early pregnancy are dependent on the specific engagement of natural cytotoxicity receptors. J Reprod Immunol. 2009;82:142–147. doi: 10.1016/j.jri.2009.06.123. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Weiss G, Moser G. Trophoblast invasion and oxygenation of the placenta: measurements versus presumptions. J Reprod Immunol. 2014;101–102:74–79. doi: 10.1016/j.jri.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006;12:137–144. doi: 10.1093/humupd/dmi043. [DOI] [PubMed] [Google Scholar]
- Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, Sukhatme VP, Karumanchi SA, Kopcow HD. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol. 2013;190:3939–3948. doi: 10.4049/jimmunol.1202582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Germeraad WT, Rouschop KM, Steeghs EM, van Gelder M, Bos GM, Wieten L. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS One. 2013;8:e64835. doi: 10.1371/journal.pone.0064835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC, Vitale M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43:2756–2764. doi: 10.1002/eji.201343448. [DOI] [PubMed] [Google Scholar]
- Whitley GS, Dash PR, Ayling LJ, Prefumo F, Thilaganathan B, Cartwright JE. Increased apoptosis in first trimester extravillous trophoblasts from pregnancies at higher risk of developing preeclampsia. Am J Pathol. 2007;170:1903–1909. doi: 10.2353/ajpath.2007.070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol. 1999;128:181–189. doi: 10.1038/sj.bjp.0702757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. The effects of oxygen concentration and gestational age on extravillous trophoblast outgrowth in a human first trimester villous explant model. Hum Reprod. 2006;21:2699–2705. doi: 10.1093/humrep/del212. [DOI] [PubMed] [Google Scholar]
- Fraser R, Whitley GS, Johnstone AP, Host AJ, Sebire NJ, Thilaganathan B, Cartwright JE. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol. 2012;228:322–332. doi: 10.1002/path.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Eastabrook G, Tan R, MacCalman CD, Dutz JP, von Dadelszen P. Decidual NK cell-derived conditioned medium enhances capillary tube and network organization in an extravillous cytotrophoblast cell line. Placenta. 2010;31:213–221. doi: 10.1016/j.placenta.2009.12.011. [DOI] [PubMed] [Google Scholar]
- El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O, Clouet-Delannoy M, Lombardelli L, Jabrane-Ferrat N, Rukavina D, Bensussan A, Piccinni MP, et al. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol. 2008;181:3009–3017. doi: 10.4049/jimmunol.181.5.3009. [DOI] [PubMed] [Google Scholar]
- Vacca P, Cantoni C, Prato C, Fulcheri E, Moretta A, Moretta L, Mingari MC. Regulatory role of NKp44, NKp46, DNAM-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int Immunol. 2008;20:1395–1405. doi: 10.1093/intimm/dxn105. [DOI] [PubMed] [Google Scholar]
- Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies–a review Placenta 2002. 23 (suppl A): S47 57 [DOI] [PubMed] [Google Scholar]
- Lash GE, Naruse K, Innes BA, Robson SC, Searle RF, Bulmer JN. Secretion of angiogenic growth factors by villous cytotrophoblast and extravillous trophoblast in early human pregnancy. Placenta. 2010;31:545–548. doi: 10.1016/j.placenta.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Engert S, Rieger L, Kapp M, Becker JC, Dietl J, Kammerer U. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am J Reprod Immunol. 2007;58:129–137. doi: 10.1111/j.1600-0897.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458–471. doi: 10.1093/humupd/dms015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta. 2009;30:320–328. doi: 10.1016/j.placenta.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139:789–798. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. 2013;13:646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MJ, Xie X, Tayade C, Peralta C, Fang Y, Leonard S, Paffaro VA, Jr, , Sheikhi AK, Murrant C, Croy BA. A review of trafficking and activation of uterine natural killer cells. Am J Reprod Immunol. 2005;54:322–331. doi: 10.1111/j.1600-0897.2005.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink T, Ebbesen P, Koppelhus U, Zachar V. Natural killer cell-mediated basal and interferon-enhanced cytotoxicity against liver cancer cells is significantly impaired under in vivo oxygen conditions. Scand J Immunol. 2003;58:607–612. doi: 10.1111/j.1365-3083.2003.01347.x. [DOI] [PubMed] [Google Scholar]
- Siewiera J, El Costa H, Tabiasco J, Berrebi A, Cartron G, Le Bouteiller P, Jabrane-Ferrat N. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog. 2013;9:e1003257. doi: 10.1371/journal.ppat.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin R, Duriez M, Berkane N, de Truchis C, Madec Y, Rey-Cuille MA, Cummings JS, Cannou C, Quillay H, Barre-Sinoussi F, Nugeyre MT, Menu E. Dynamic shift from CD85j/ILT-2 to NKG2D NK receptor expression pattern on human decidual NK during the first trimester of pregnancy. PLoS One. 2012;7:e30017. doi: 10.1371/journal.pone.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Percival K, Searle RF, Robson SC, Bulmer JN. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod. 2010;25:1137–1145. doi: 10.1093/humrep/deq050. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao A, Wang X, Shi G, Jin H, Lin Q. Expressions of natural cytotoxicity receptors and NKG2D on decidual natural killer cells in patients having spontaneous abortions. Fertil Steril. 2008;90:1931–1937. doi: 10.1016/j.fertnstert.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Mei B, Zhang SR, Chen YL, Wang CF. Defects in NKG2D ligand expression result in failed tolerance induction at the maternal-fetal interface: a possible cause for recurrent miscarriage. Med Hypotheses. 2012;79:465–467. doi: 10.1016/j.mehy.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, Darretta V, Moretta L, Mingari MC. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U S A. 2011;108:2402–2407. doi: 10.1073/pnas.1016257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clin Exp Immunol. 2005;139:287–296. doi: 10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]