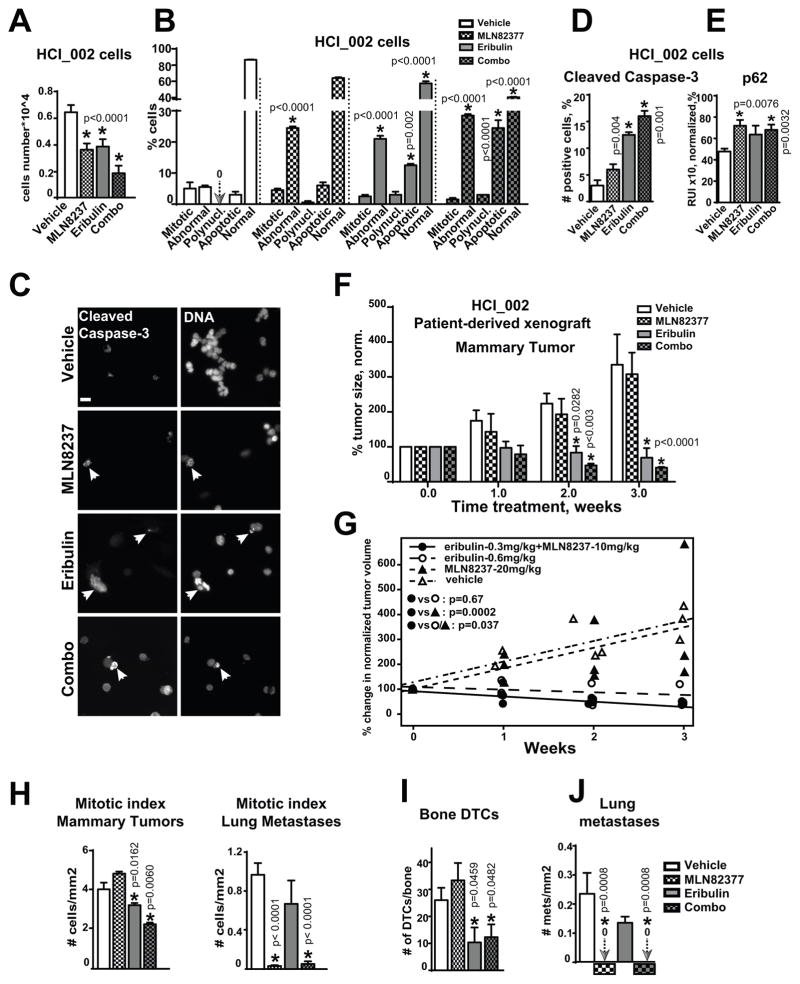
Figure 6. MLN8237+eribulin reduces primary tumor, metastases and dissemination of cancer in patient-derived xenograft models.
A HCI_002 cell growth analysis, 48h; vehicle, 100nM-MLN8237, 3nM-eribulin, or combination; n=3, one-way ANOVA: vehicle vs. treatments. B Analysis of the nuclear morphology, cells as in (A); one-way ANOVA: vehicle vs. treatments in each category: mitotic, abnormal nucleus, polynucleated, apoptotic, normal. C Representative IF images of HCI_002 cells treated as in (A) with anti-cleaved-caspase-3/green antibodies, DNA, scale bar-20μm; white arrow identifies apoptotic DNA. D Quantification of cleaved-caspase-3 positive cells using IF images as in (C); one-way-ANOVA: vehicle vs. treatments. E IF-based analysis of cells as in (A) with p62 antibodies; the relative intensity (RIU) of the IF signal from 3 independent experiments; 100 cells/treatment, one-way-ANOVA: vehicle vs. treatments. F Tumor volume analysis of HCI_002-PDX mammary xenografts in three cohorts, n=6/cohort; Tumor growth was normalized to time point zero of treatment (100%) in each cohort; one-way ANOVA: vehicle vs. treatments. G Statistical analysis of the data as in F using mixed model; H Mitotic index analysis of metastases/lungs or mammary tumors as in (F), n=50 cells/treatment in 3 experiments, t-test. I Analysis of disseminated tumor cells in femurs of mice as in (F); n=2/mouse; one-way ANOVA: vehicle vs. treatments. J Quantification of lung metastases; serial sections of lungs from three mice/treatment, one-way ANOVA: vehicle vs. treatments. MLN8237 and combination treated mice did not have metastases; arrows and zero signs visualize the absence of signal.