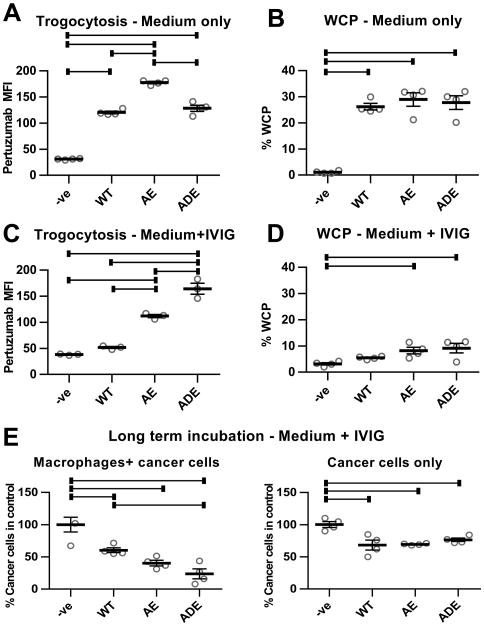
Figure 5.
Antibodies with enhanced affinity for activating FcγRs have increased trogocytic and cell-killing activity. A, thioglycollate-elicited macrophages isolated from C57BL/6 mice transgenically expressing human FcγRs (hFcγR macrophages; 26) were plated with MDA-MB-453 cells at an effector:target ratio of 0.4:1 (4×104:1×105 cells). 18 hours later, 1 μg/ml wild type (WT) trastuzumab or engineered trastuzumab variants with enhanced affinity for FcγRIIa and FcγRIIIa (AE and ADE), combined with 0.5 μg/ml Alexa 488-labeled Fab fragments derived from pertuzumab, were added to the co-cultures for 60 minutes. As controls, cells were also co-cultured without trastuzumab (-ve). The mean fluorescence intensity (MFI) values for pertuzumab Fab staining in the macrophage population are shown. B, WCP assay was performed as before using hFcγR macrophages plated for 18 hours, followed by addition of 10-fold lower numbers of cancer cells and 10 μg/ml WT, AE or ADE trastuzumab variants for 6 hours. Trogocytosis (C) and WCP (D) assays were performed as in A,B, but in the presence of 10 mg/ml IVIG. Under the conditions of the trogocytosis assays (60 minutes incubation), phagocytosis of cancer cells was at background, control levels (data not shown). E, hFcγR macrophages were plated with cancer cells at an effector:target cell ratio of 2:1 (5×104:2.5×104 cells) or cancer cells alone for 24 hours, followed by addition of 10 mg/ml IVIG and 10 μg/ml WT, AE or ADE variants of trastuzumab. The medium was replaced by new medium containing the same additions after 3 days. Cells were harvested after 5 days and the remaining numbers of cancer cells quantitated. Error bars represent standard errors. For A-E, one-way ANOVA analyses were carried out followed by a Tukey's multiple comparisons test between all sample pairs with a confidence interval of 95%. Horizontal lines indicate significant differences between sample pairs.