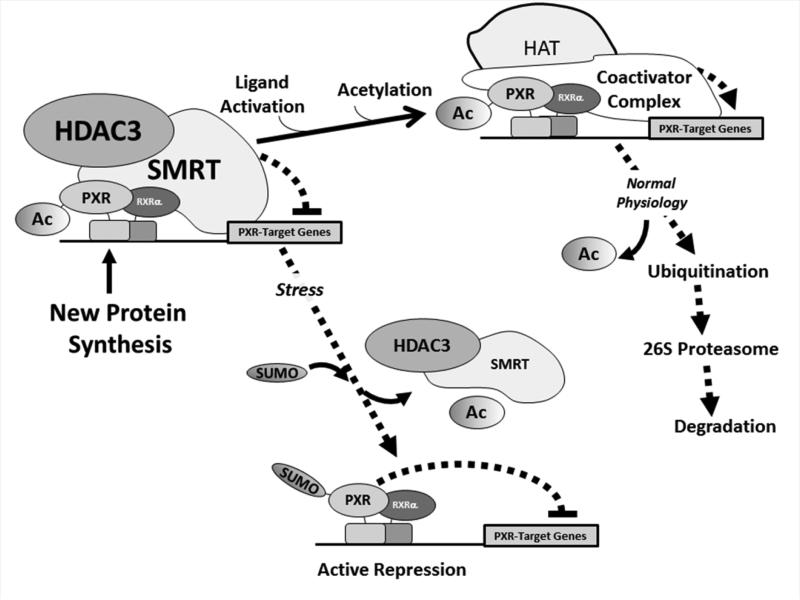
Figure 9. Working model of the Role of the Acetyl-SUMO Switch in PXR Biology.
Newly synthesized PXR protein is acetylated and poised on canonical PXR-target genes in a complex with the HDAC3-SMRT co-repressor multi-protein complex and is transcriptionally silent. Ligand activation promotes hyper-acetylation of the genomic locus, likely through the action of canonical histone/lysine acetyltransferase enzymes belonging to the E1A binding protein p300/CREB-binding protein coactivator family. Following one round of transcription the PXR-associated multi-protein complex is degraded by the 26S proteasome in an ubiquitin-dependent manner, and the promoter is thus cleared and poised to receive another round of transcriptional machinery. In the presence of specific signals, such an inflammatory stress or potentially other extra-cellular stimuli, PXR is de-acetylated and the HDAC3-SMRT co-repressor multi-protein complex is disassociated. The resulting signal-dependent action of a SUMO E3 ligase enzyme, such as PIAS1, promotes PXR-SUMOylation to inhibit PXR-target gene expression in an acetylation-dependent manner.