Abstract
Background
Cervical dystonia (CD) is a debilitating neurological disorder that may gravely affect a patient’s quality of life (QoL). Botulinum toxin treatment has been approved as a first-line treatment for this condition. This study aims to look at the efficacy and impact on the QoL of neu-botulinumtoxinA, a newer and cheaper botulinum toxin type A, in patients with CD.
Methods
This is a prospective, open-label, single-arm study. CD patients were recruited and evaluated for severity of CD using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), and for QoL using the Craniocervical Dystonia Questionnaire (CDQ-24), and the 36-item Short Form Health Survey questionnaire (SF-36) at baseline and 6 weeks after injection.
Results
Twenty patients were recruited. Significant improvement was shown in part 1 and total TWSTRS score and total CDQ-24 scores. Analysis of individual items of the TWSTRS scale showed significant improvement in rotation, duration of CD, and work ability. Significant improvements in the QoL were also seen in some items of the stigma, emotional wellbeing, and energy/fatigue domains of the CDQ-24 and SF-36 questionnaires.
Discussion
Neu-botulinumtoxinA is efficacious in treating CD symptoms and improving QoL of patients with CD. A larger, double-blinded study is needed to study the extent of improvements.
Keywords: Cervical dystonia, neu-botulinumtoxinA, botulinum toxin, quality of life
Introduction
Cervical dystonia (CD) is a neurological condition in which excessive involuntary muscle contraction causes abnormal posture and movement of the head and neck.1,2 It may be accompanied by pain and head tremor. These can cause disability, a negative self-image, and decreased quality of life (QoL).3–6 Apart from these, other factors, such as depression, ability to cope with the disease, severity and duration of dystonia, female gender, stigma, and poor financial situation, also play significant roles in determining the QoL of CD patients.4,7–10 The negative impact of CD can be significant and is similar to Parkinson’s disease, multiple sclerosis, and stroke.5,11
Botulinum toxin (BTX) injection, especially BTX type A (BTX-A), has been accepted as the treatment of choice for patients with CD. Neu-botulinumtoxinA (N-BTX, Neuronox®) is a relatively new BTX-A that has been approved for various therapeutic and cosmetic indications in some Asian and Latin American countries. It is produced by the Hall A strain of Clostridium botulinum and purified to produce a homogeneous 900-kD toxin complex. It has been shown to be non-inferior to onabotulinumtoxinA (Ona-BTX, Botox®) in the treatment of blepharospam, moderate to severe glabellar lines, post-stroke upper limb spasticity, and spastic equinus gait in children with cerebral palsy.12–16 Its cost is lower than Ona-BTX and abobotulinumtoxinA (Abo-BTX, Dysport®) by approximately 10–30%.
The purpose of this study was to investigate the efficacy and impact on the QoL of N-BTX treatment in CD patients.
Methods
This was a prospective, open label, single-arm study. The primary objective of the study was to study the efficacy of N-BTX on the severity of CD. The secondary objective was to study its impact on the QoL of patients with CD. The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University. Patients with CD were recruited from the BTX clinic of Chulalongkorn Center of Excellence for Parkinson’s Disease and Related Disorders. Patients with CD who were ≥18–75 years old were eligible to participate in the study. Patients were excluded if they were in any of these categories: patients who had been treated with BTX-A within 3 months; females who were pregnant, planning pregnancy, unable to use contraception, or lactating; and any medical condition that may have put the subject at increased risk with exposure to BTX at the discrimination of investigators.
If patients were taking concomitant oral medications for dystonia, the medicines and doses were left unchanged during the study period.
Patients were evaluated using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), a specific scale to rate cervical dystonia divided into severity, disability, and pain subscales; the Craniocervical Dystonia Questionnaire (CDQ-24), a disease-specific questionnaire to evaluate QoL of patients with CD and blepharospasm based on five subscales: stigma, emotional wellbeing, pain, activities of daily living (ADL), and social/family life17; and the 36-item Short Form Health Survey questionnaire (SF-36), which is a generic measure of QoL18,19 looking at eight domains: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional wellbeing, social functioning, pain, and general health. These scales were administered at baseline and 6 weeks after injection. At 6 weeks, the subject’s overall satisfaction was also evaluated by asking how satisfied they were with the treatment (very unsatisfied, unsatisfied, slightly unsatisfied, not so bad, slightly satisfied, satisfied, and very satisfied).
Patients were evaluated by TWSTRS and injected according to the type of CD by movement disorders neurologists. The doses were based on prior BTX treatment with other types of toxins and adjusted according to the patient’s current symptoms. The dose conversion ratio for Ona-BTX was 1:1 and for Abo-BTX was 1:2–3.12,14,20 The CDQ-24, SF-36 questionnaires, and the subject’s overall satisfaction scale were self-administered by the patients or the questions were asked by a movement disorders nurse when the patients had difficulty reading or were uneducated and the answers were provided by the patients.
Statistics
For demographics, disease characteristics, and the subject’s overall satisfaction, descriptive statistics were used. Outcome variables were evaluated using the Wilcoxon Signed Rank Test. A p-value ≤0.05 was considered statistically significant.
Results
A total of 20 patients were enrolled in the study. Baseline demographics and disease characteristics are shown in Table 1. Two of the 20 patients had secondary CD, one from cerebral palsy and the other from iron accumulation in the basal ganglia from an unidentified cause. Their diseases were non-progressive or very slowly progressive over the past 5 years of follow-up. The mean last dose of Ona-BTX before entering the study was 81.04 units (range 0–262 units, SD ± 77.3) and of Abo-BTX was 394 units (range 210–500 units, SD ± 129.6). All the patients had at least one concomitant oral medication for dystonia. These included anticholinergics, baclofen, clonazepam, levodopa, sirdalud, gabapentin, and pregabalin. The doses were left unchanged during the study period.
Table 1. Baseline Demographics and Disease Characteristics.
| Demographic Variables | Study Population (n = 20) |
|---|---|
| Gender: male, n (%) | 11 (55) |
| Mean (range) age, years | 45.8 (24–73) |
| Mean (range) age at onset, years | 38.8 (7–67) |
| Mean (range) estimated duration of cervical dystonia, years | 7.0 (1–19) |
| Pain at onset, n (%) | 9 (45.0) |
| Tremor at onset, n (%) | 11 (55.0) |
| Jerk at onset, n (%) | 3 (15.0) |
| Sensory tricks at onset, n (%) | 5 (25.0) |
| Clinical presentation, n (%) | |
| Simple cervical dystonia | |
| Torticollis | 5 (25) |
| Laterocollis | 0 (0) |
| Retrocollis | 2 (10) |
| Anterocollis | 1 (5) |
| Complex cervical dystonia | |
| Torticollis + laterocollis | 6 (30) |
| Torticollis + retrocollis | 1 (5) |
| Torticollis + anterocollis | 1 (5) |
| Torticollis + laterocollis + retrocollis | 1 (5) |
| Torticollis + laterocollis + anterocollis | 3 (15) |
| Number of injections before entering the study | |
| Never, n (%) | 1 (5) |
| 1–5, n (%) | 10 (50) |
| 6–10, n (%) | 1 (5) |
| 11–15, n (%) | 4 (20) |
| 16–25, n (%) | 4 (20) |
| Duration of injection before entering the study (years) | |
| Never, n (%) | 1 (5) |
| 1–5, n (%) | 13 (65) |
| 6–10, n (%) | 5 (25) |
| 11–15, n (%) | 1 (5) |
| Previous Ona-BTX injection, n (%) | 14 (70) |
| Previous Abo-BTX injection, n (%) | 6 (30) |
| Previous Ona-BTX and Abo-BTX injection, n (%) | 2 (10) |
Abbreviations: Abo-BTX, AbobotulinumtoxinA; Ona-BTX, OnabotulinumtoxinA.
The mean dose of N-BTX injected was 120 units (range 30–300 units, SD ± 69). Part 1 (severity) and the total TWSTRS scores improved significantly post-injection (p = 0.006 and 0.010 respectively). Analysis of individual points of the TWSTRS scale revealed significant changes in rotation (p = 0.008), duration factor (p = 0.003), and work ability (p = 0.047) (part 2) scores. The results of other outcomes are shown in Table 2.
Table 2. Outcome Variables.
| Outcome Measure | Mean score (n = 20) | p | ||
|---|---|---|---|---|
| Baseline | 6 Weeks after Injection | |||
| TWSTRS | Part 1: severity (max = 35)1 | 19.55 | 16.5 | 0.006 |
| Part 2: disability (max = 30)1 | 6.5 | 5.1 | 0.210 | |
| Part 3: pain (max = 20)1 | 6.65 | 5.64 | 0.322 | |
| Total score (max = 85)1 | 26.05 | 21.60 | 0.010 | |
| CDQ-24 (max = 100)1 | 29.85 | 25.53 | 0.050 | |
| SF-36 (max = 100)1,2 | 56.26 | 59.63 | 0.709 | |
Abbreviations: CDQ-24, Craniocervical Dystonia Questionnaire; SF-36, Craniocervical Dystonia Questionnaire; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale.
Maximum possible score.
Higher SF-36 score indicates better health.
Analysis of each of the domains of SF-36 and CDQ-24 did not show significant difference before and after the treatment. However, the total CDQ-24 score was significantly different (p = 0.05). Detailed analysis of the CDQ-24 and SF-36 questionnaires revealed that questions 12 and 22 of the CDQ-24, “have you felt afraid” (emotional wellbeing) and “have you felt you didn’t look so good” (stigma), respectively, and questions 23 and 31 of the SF-36 questionnaire, “did you feel full of pep” and “did you feel tired”, respectively, both of which are in the energy/fatigue domain, were statistically significantly different at baseline and 6 weeks after injection (p-values of 0.035, 0.022, 0.036 and 0.033, respectively).
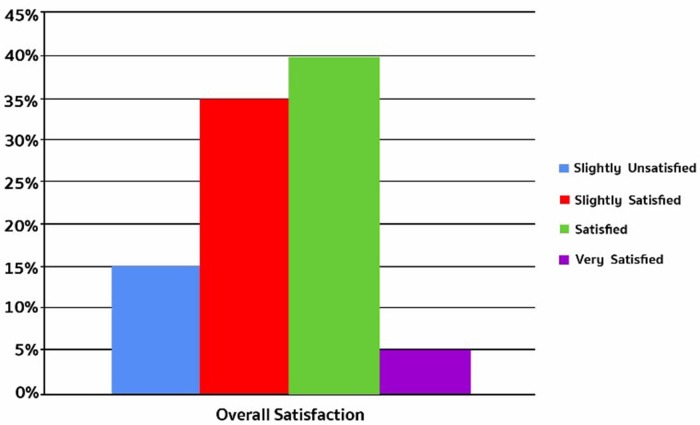
At 6 weeks, most patients were either satisfied (40%) or slightly satisfied (35%) with the result of the injection (Figure 1).
Figure 1. Patients’ overall satisfaction at 6 weeks post-injection.
There was no major adverse event from the injection. One patient complained of headache after injection that was resolved by acetaminophen. The others did not complain of any adverse events.
Discussion
This is the first study of N-BTX in CD patients. It shows that N-BTX is efficacious in treating symptoms of CD shown by significant changes in part 1 and total TWSTRS scores. The efficacy was more prominent in rotation and duration of CD reduction and increase in work ability. The total score of CDQ-24, a QoL questionnaire specifically designed for CD patients, significantly improved after N-BTX injection. The total SF-36 score was not significantly different, but some parts of both the SF-36 and the CDQ-24 questionnaires in stigma, emotional wellbeing, and energy/fatigue domains show statistically significant improvement. This may imply that when the motor symptoms of CD improve, some aspects of energy level and self-perception are more sensitive to change than other aspects and domains, and that these changes can be brought about by BTX treatment. Most of the patients were satisfied with the outcome of the treatment. This is in line with the study by Sethi et al.21 that looked into CD patients’ satisfaction with BTX treatment that revealed that most patients were satisfied with their treatment and about 6.6% were unsatisfied.
Previous studies looking at the QoL of CD patients have shown that BTX-A treatment improves the patients’ QoL and TWSTRS or Tsui scores, as early as 4 weeks after the first injection, and this increases with the duration of treatment.5–7,9,17,22 In the largest, prospective, open-label study of 516 CD patients by Hefter et al.,6 there were significant changes in the total and subscale scores of the CDQ-24 pain and day-to-day capacity at week 4 that was sustained at week 12 after Abo-BTX treatment. Another study by Skogseid et al.9 looking at the QoL of 70 CD patients (median duration of BTX treatment 5.5 years) concluded that most CD patients enjoy a good QoL after long-term BTX-A therapy. Slawek et al.7 studied the impact of BTX-A on the QoL in 101 CD patients, and improvement was seen in all the SF-36 domains. Smaller studies, however, have shown improvement in only some domains. Mordin et al.5 analyzed the effect of Abo-BTX on QoL in 45 CD patients compared with 38 controls. The SF-36 improved in certain domains: physical functioning, role limitations because of physical health, bodily pain, general health, and role limitations because of emotional problems. Another smaller study by Hilker et al.22 (25 CD patients) showed improvement in energy and vitality, social functioning, mental health (emotional wellbeing), limitations because of physical problems, and pain domains of the SF-36. This may explain why there were no significant differences in individual domains of the two QoL questionnaires in this study. The effect seems to be more prominently evident with the increased number of patients recruited in the studies. As this was a small study, the effect may not have been revealed.
Another explanation for the lack of statistically significant improvement in the QoL and other symptoms of patients in the study may be due to patient profiles. Most of the patients that entered the study did not have much disability, pain, and poor QoL specifically caused by CD, as the baseline scores of part 2 and 3 of TWSTRS and total CDQ-24 were not high. Moreover, the patients were quite diversified, as shown by wide standard deviation (SD) of the mean baseline total TWSTRS score (SD = 10.185) and mean baseline total CDQ-24 score (SD = 20.671). These may be the reasons for the above scores not being significantly different after treatment.
There are some limitations of this study. Firstly, this was an open-label study and the number of patients recruited was small. Patients were quite diversified. Most of them did not have much pain and disease-specific poor QoL. Depression was not assessed in the study and, as mentioned above, it is one of the major predictors of the QoL of patients with CD.7,8 Additionally, the doses of N-BTX used in the study were quite low as they were converted from the previous BTX doses that the patients had been injected with. The dosage of BTX was limited by the reimbursement scheme in the country of the study. For CD, patients can be reimbursed for not more than 300 units of Ona-BTX per year and not more than 500 units of Abo-BTX per year. Those who are willing to pay by themselves usually get higher doses. Therefore, the toxin is injected into the most troublesome muscles with the limitation of the dose in mind. A larger and a double-blind study that includes patients with more disability, pain, and a poorer QoL would be needed to prove the efficacy of N-BTX at improving the non-motor symptoms and the QoL of patients with CD. Using the outcome of this study, the sample size that would be required would be at least 89.
In conclusion, N-BTX is a cheaper alternative and is efficacious in treating the symptoms of CD and helps improve a patient’s QoL. A larger, double-blind study is needed to confirm the result.
Footnotes
Funding: The study was supported by Medytox, Korea, and Celeste, Thailand.
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors' institutional ethics committee has approved this study and all patients have provided written informed consent.
References
- 1.Jinnah HA, Berardelli A, Comella C, et al. The focal dystonias: current views and challenges for future research. Mov Disord. 2013;28:926–943. doi: 10.1002/mds.25567. doi: 10.1002/mds.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albanese A, Abbruzzese G, Dressler D, et al. Practical guidance for CD management involving treatment of botulinum toxin: a consensus statement. J Neurol. 2015;262:2201–2213. doi: 10.1007/s00415-015-7703-x. doi: 10.1007/s00415-015-7703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werle RW, Takeda SY, Zonta MB, Guimaraes AT, Teive HA. The physical, social and emotional aspects are the most affected in the quality of life of the patients with cervical dystonia. Arq Neuropsiquiatr. 2014;72:405–410. doi: 10.1590/0004-282x20140044. doi: 10.1590/0004-282X20140044. [DOI] [PubMed] [Google Scholar]
- 5.Mordin M, Masaquel C, Abbott C, Copley-Merriman C. Factors affecting the health-related quality of life of patients with cervical dystonia and impact of treatment with abobotulinumtoxinA (Dysport): results from a randomised, double-blind, placebo-controlled study. BMJ Open. 2014;4:e005150. doi: 10.1136/bmjopen-2014-005150. doi: 10.1136/bmjopen-2014-005150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hefter H, Benecke R, Erbguth F, Jost W, Reichel G, Wissel J. An open-label cohort study of the improvement of quality of life and pain in de novo cervical dystonia patients after injections with 500 U botulinum toxin A (Dysport) BMJ Open. 2013;3:1–7. doi: 10.1136/bmjopen-2012-001853. doi: 10.1136/bmjopen-2012-001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slawek J, Friedman A, Potulska A, et al. Factors affecting the health-related quality of life of patients with cervical dystonia and the impact of botulinum toxin type A injections. Funct Neurol. 2007;22:95–100. [PubMed] [Google Scholar]
- 8.Ben-Shlomo Y, Camfield L, Warner T, ESDE Collaborative Group What are the determinants of quality of life in people with cervical dystonia? J Neurol Neurosurg Psychiatry. 2002;72:608–614. doi: 10.1136/jnnp.72.5.608. doi: 10.1136/jnnp.72.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skogseid IM, Malt UF, Roislien J, Kerty E. Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur J Neurol. 2007;14:1129–1137. doi: 10.1111/j.1468-1331.2007.01922.x. doi: 10.1111/j.1468-1331.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- 10.Queiroz MR, Chien HF, Barbosa ER. Quality of life in individuals with cervical dystonia before botulinum toxin injection in a Brazilian tertiary care hospital. Arq Neuropsiquiatr. 2011;69:900–904. doi: 10.1590/s0004-282x2011000700010. doi: 10.1590/S0004-282X2011000700010. [DOI] [PubMed] [Google Scholar]
- 11.Camfield L, Ben-Shlomo Y, Warner TT, Epidemiological Study of Dystonia in Europe Collaborative Group Impact of cervical dystonia on quality of life. Mov Disord. 2002;17:838–841. doi: 10.1002/mds.10127. doi: 10.1002/mds.10127. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JS, Kim JC, Lee SY. Double-blind, randomized, comparative study of Meditoxin versus Botox in the treatment of essential blepharospasm. Korean J Ophthalmol. 2009;23:137–141. doi: 10.3341/kjo.2009.23.3.137. doi: 10.3341/kjo.2009.23.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Won CH, Lee HM, Lee WS, et al. Efficacy and safety of a novel botulinum toxin type A product for the treatment of moderate to severe glabellar lines: a randomized, double-blind, active-controlled multicenter study. Dermatol Surg. 2013;39:171–178. doi: 10.1111/dsu.12072. doi: 10.1111/dsu.12072. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Shin HI, Kwon BS, Kim SJ, Jung IY, Bang MS. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: a randomized, double-blinded, controlled multicentre clinical trial. Dev Med Child Neurol. 2011;53:239–244. doi: 10.1111/j.1469-8749.2010.03830.x. doi: 10.1111/j.1469-8749.2010.03830.x. [DOI] [PubMed] [Google Scholar]
- 15.Nam HS, Park YG, Paik NJ, et al. Efficacy and safety of NABOTA in post-stroke upper limb spasticity: a phase 3 multicenter, double-blinded, randomized controlled trial. J Neurol Sci. 2015;357:192–197. doi: 10.1016/j.jns.2015.07.028. doi: 10.1016/j.jns.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Seo HG, Paik NJ, Lee SU, et al. Neuronox versus BOTOX in the treatment of post-stroke upper limb spasticity: a multicenter randomized controlled trial. PLoS One. 2015;10:e0128633. doi: 10.1371/journal.pone.0128633. doi: 10.1371/journal.pone.0128633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller J, Wissel J, Kemmler G, et al. Craniocervical dystonia questionnaire (CDQ-24): development and validation of a disease-specific quality of life instrument. J Neurol Neurosurg Psychiatry. 2004;75:749–753. doi: 10.1136/jnnp.2003.013441. doi: 10.1136/jnnp.2003.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leurmarnkul W, Meetam P. Properties testing of the retranslated SF-36 (Thai Version) Thai J Pharm Sci. 2005;29:69–88. [Google Scholar]
- 19.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung ME, Song DH, Park JH. Comparative study of biological activity of four botulinum toxin type A preparations in mice. Dermatol Surg. 2013;39:155–164. doi: 10.1111/dsu.12071. doi: 10.1111/dsu.12071. [DOI] [PubMed] [Google Scholar]
- 21.Sethi KD, Rodriguez R, Olayinka B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J Med Econ. 2012;15:419–423. doi: 10.3111/13696998.2011.653726. doi: 10.3111/13696998.2011.653726. [DOI] [PubMed] [Google Scholar]
- 22.Hilker R, Schischniaschvili M, Ghaemi M, Jacobs A, Rudolf J. Health related quality of life is improved by botulinum neurotoxin type A in long term treated patients with focal dystonia. J Neurol Neurosurg Psychiatry. 2001;71:193–199. doi: 10.1136/jnnp.71.2.193. doi: 10.1136/jnnp.71.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]