Abstract
Prospective memory (PM) is associated with antiretroviral (ARV) adherence in HIV, but little is known about how pill burden and age might affect this association. 117 older (≥ 50 years) and 82 younger (< 50 years) HIV-infected adults were administered a measure of PM in the laboratory and subsequently were monitored for ARV adherence for 30 days using the Medication Event Monitoring System. In the older group, better time-based PM performance was associated with higher likelihood of adherence, irrespective of pill burden. Within the younger sample, time-based PM was positively related to adherence only in participants with lower pill burdens. Younger HIV-infected individuals with higher pill burdens may overcome the normal effects of time-based PM on adherence through compensatory medication taking strategies, while suboptimal use of these strategies by younger HIV-infected individuals with lower pill burdens may heighten their risk of ARV non-adherence secondary to deficits in time-based PM.
Keywords: adherence, aging, HIV, pill burden, prospective memory
The prevalence of older adults (ages ≥ 50 years) living with HIV disease has been predicted to rise to 75% by 2030 (Smit et al., 2015). This increased number of older HIV-infected adults is in part due to enhanced longevity secondary to the increasingly widespread use and effectiveness of combined antiretroviral therapies (cART), as well as higher incidence rates in this age range (Centers for Disease Control and Prevention, 2015). Older age in the context of HIV infection is associated with a higher likelihood of having HIV-associated, Non-AIDS conditions (e.g., cardiovascular disease) and other medical comorbidities (e.g., diabetes), which has given rise to a commensurate increase in the number of medications prescribed to older HIV-infected adults (Krentz, Cosman, Lee, Ming, & Gill, 2012). Older HIV-infected adults are estimated to take more than seven total medications per day (Krentz et al., 2012), which is considerably higher than their younger HIV-infected (Zhou et al., 2014) and older uninfected counterparts (Lee et al., 2012). The increase in medication burden for older HIV-infected adults has sparked concerns about undue polypharmacy and subsequent risks of non-adherence (Nachega, Hsu, Uthman, Spinewine, & Pham, 2012).
Non-adherence to antiretroviral (ARV) medications is a significant barrier to adequate management of HIV infection and leads to poorer health outcomes, including viremia (Li et al., 2014), comorbidity burden (Sherr et al., 2010), and risk of mortality (Glass et al., 2015). Despite more than 25 years of research, a high rate of ARV non-adherence in HIV-infected individuals remains. It has been estimated that more than 12% of individuals miss more than 90% of ARV doses (Glass et al., 2015). Although non-adherence is clearly multi-determined, there has been interest in the role that medication regimens may play in this critical health behavior (Maggiolo et al., 2002). For example, lower ARV pill burden is broadly related to better medication adherence and improved health outcomes (O’Connor et al., 2013). As such, the complexity and burden of ARV medication regimens have been reliably reduced since the initial introduction of cART (Krentz et al., 2012). However, the persistence of non-adherence in the era of increasingly simplified ARV regimens highlights the need to more closely investigate the potential modulators of the relationship between pill burden and adherence across the lifespan.
While older age is generally associated with better medication adherence in HIV (Ettenhofer et al., 2009), older adults with neurocognitive impairment are at a disproportionate risk for ARV non-adherence (Hinkin et al., 2004; cf. Vance, Fazeli, & Gakumo, 2013). This is important because older HIV-infected adults are also at greater risk for HIV-associated neurocognitive disorders (HAND); for example, a study by Ettenhofer et al. (2009) found that poorer neuropsychological functioning (i.e., executive functions, motor skills, and information processing speed) was associated with lower ARV adherence in older, but not younger HIV-infected adults. Similarly, older adults have appeared to be more vulnerable than their younger counterparts to the adverse impact of HAND on retention in HIV care (Jacks et al., 2015) and medication management capacity (Thames et al., 2011). Taken together, the increased medication burden and neurocognitive difficulties often faced by older HIV-infected adults raises the possibility that these two factors may interact to adversely affect health behaviors, such as ARV adherence.
One cognitive ability that may play an especially important role in medication adherence in HIV-infected persons is prospective memory (PM). Colloquially known as “remembering to remember,” PM is a unique aspect of memory that describes one’s ability to form, retain, and successfully execute a future intention. Common examples of PM include remembering to take a medication as scheduled or remembering to attend a health care appointment. PM is a complex function that involves: (a) Initially forming an intention to do something at a later occasion in response to a specific cue (e.g., take a medication as prescribed at 8:00 p.m.); (b) Retaining that intention in the face of another ongoing activity (e.g., daily life); (c) Watching for and noticing the cue that indicates the intention should be executed (e.g., monitoring the clock and recognizing that it is 8:00 p.m.); (d) Recalling the intention from retrospective memory (e.g., the need to take the specific medication and under what conditions; and (e) Successfully executing the intention (e.g., taking the medication as prescribed). As is clear from this example, PM is a multifaceted ability that places demands on other higher-level cognitive functions, including retrospective memory (i.e., recall of events and information from the past) and executive functions (e.g., planning and cognitive flexibility). However, PM is a unique constellation of these component cognitive processes and is separable from them at the cognitive (Gupta, Woods, Weber, Dawson, & Grant, 2010), neurobiological (Woods et al., 2006), and functional (e.g., Woods, Moran, Carey, et al., 2008) levels. In addition, there are numerous variables that have been shown to have a modulating effect on both PM as well as adherence, including compensatory strategy use (Blackstone, Woods, Weber, Grant, & Moore, 2013; Weber et al., 2011), depression (Li, Weinborn, Loft, & Maybery, 2013; Poquette et al., 2013), and disease severity (Patton et al., 2012). As such, examining PM as a possible predictor of adherence in the context of increased demands placed by higher medication burdens in the everyday lives of older adults may be particularly important.
Consistent with reports that forgetting is the most commonly cited reason for non-adherence in HIV-infected adults (Kalichman, Cain, Cherry, Kalichman, & Pope, 2005; Liu et al., 2014), HIV-associated deficits in PM are independently associated with medication non-adherence (Woods, Moran, Carey, et al., 2008; Zogg, Woods, Sauceda, Wiebe, & Simoni, 2012). Two different types of PM are important to understanding the role of this cognitive ability area in medication adherence for HIV-infected persons: (a) time-based PM (e.g., remembering to take a medication at 8:15 a.m.) and (b) event-based PM (e.g., remembering to take a medication with breakfast). Time-based PM is thought to be more strategically demanding than event-based PM. Research to date has shown that deficits in time-based PM are strongly and uniquely associated with medication non-adherence in HIV disease (Poquette et al., 2013; Woods et al., 2009). Critically, older HIV-infected adults are vulnerable to deficits in strategically demanding time-based PM (Woods, Dawson, Weber, & Grant, 2010), including time-based PM tasks (Weber et al., 2011). Thus, it is possible that the increased strategic real-world demands of a more complex and burdensome medication regimen may exacerbate the adverse effects of time-based PM deficits on ARV adherence in older adults. Determining the possible effects of pill burden and age on time-based PM performance and consequent medication adherence in HIV may allow for nurses in clinical practice to better predict which patients might be at higher risk for non-adherence. Further, identifying cognitive risk factors (e.g., prospective memory) associated with poor adherence in the context of high pill burden and older age may highlight a subgroup within the HIV-infected population that could disproportionately benefit from compensatory training programs implemented in clinical practice.
Our study, therefore, sought to examine the influence of pill burden on the relationship between time-based PM and ARV adherence in older versus younger HIV-infected adults. We hypothesized that a higher pill burden would heighten the deleterious effects of time-based PM on non-adherence, especially for older versus younger adults. In other words, we expected that older HIV-infected adults’ greater pill burden and regimen complexity would amplify environmental demands and increase the opportunity for diminished PM capacity to interfere with medication taking behaviors.
Methods
Participants
The institutional human research protections review board at the University of California, San Diego approved the study. Older (≥ 50 years; n = 117) and younger (< 50 years; n = 82) HIV-infected adults were drawn retrospectively from two NIH-funded observational cohort studies that measured ARV adherence using a 30-day medication event monitoring system (MEMS) approach (described below).
Exclusion criteria consisted of having of a diagnosis of a psychotic disorder (e.g., schizophrenia), a neurological disease associated with cognitive impairment (e.g., traumatic brain injury with a loss of consciousness > 5 min), or an estimated verbal IQ lower than 70 (as determined by the Wechsler Test of Adult Reading). Additionally, participants were excluded if they met criteria for substance dependence within 1 month prior to evaluation (as determined by the Composite International Diagnostic Interview, version 2.1), had a Breathalyzer test positive for alcohol on date of testing, or had a urine toxicology screening test on the day of evaluation that was positive for illicit drugs (except for marijuana). Inclusion criteria consisted of being infected with HIV (diagnosed with both enzyme linked immunosorbent assay as well as a Western Blot test or a rapid test), capacity to provide written, informed consent on date of testing, and prescription of at least one ARV medication. Table 1 shows the disease, demographic, and psychiatric characteristics of the study samples.
Table 1.
Mean (Standard Error) Demographic and Disease Characteristics
| Variable | Older HIV Infected (n = 117) |
Younger HIV Infected (n = 82) |
|---|---|---|
| Demographic | ||
| Age (years)* | 56.7 (0.6)* | 37.1 (0.7)* |
| Education (years)* | 14.0 (0.2)* | 12.8 (0.3)* |
| Gender (% male) | 85.5 | 87.8 |
| Estimated Verbal IQ (WTAR) | 103.3 (1.0) | 101.6 (1.3) |
| Ethnicity (%) | ||
| Caucasian* | 72.7* | 52.4* |
| African American | 17.1 | 26.8 |
| Hispanic* | 9.4* | 20.7* |
| Native American | 0.9 | 0.0 |
| Psychiatric/Neuropsychiatric | ||
| HIV-Associated Neurocognitive Impairment (%) | 26.1 | 23.5 |
| POMS Total Mood Disturbance (of 200) | 58.4 (3.4) | 56.4 (4.3) |
| Lifetime Major Depressive Disorder (%) | 53.9 | 56.1 |
| Current Major Depressive Disorder (%) | 11.1 | 11.0 |
| Lifetime Generalized Anxiety Disorder (%) | 17.2* | 3.7* |
| Current Generalized Anxiety Disorder (%) | 4.3 | 1.2 |
| Lifetime Substance Dependence (%) | 53.0 | 54.3 |
| Medical | ||
| Hepatitis C virus (%)* | 29.1* | 11.0* |
| Current CD4+ T cell count | 573.3 (28.8) | 573.4 (30.1) |
| Nadir CD4+ T cell count* | 165.7 (14.8)* | 199.5 (15.9)* |
| Plasma RNA detectable (%) | 10.53 | 20.00 |
| AIDS diagnosis (%) | 70.1 | 61.0 |
| Duration of infection (years)* | 17.2 (0.6)* | 11.3 (0.8)* |
Note. WTAR = Wechsler Test of Adult Reading; POMS = Profile of Mood States;
p < .05.
Prior to evaluation, all participants provided informed, written consent. Participants then completed evaluations assessing neuropsychological, psychiatric, and medical history. All participants received financial compensation of $35 USD for participation in the 3-hour neurocognitive aspect of this study.
Neuromedical Assessment
As part of a lifetime medical history interview conducted by a research nurse, participants were asked how many medications they were currently taking, including ARV and non-ARV medications. Total number of pills per day was calculated for ARV and non-ARV medications separately (see Table 2). In either case, pill burden was classified into High or Low based on a median split (median total medications = 7; low group ≤ 7 pills).
Table 2.
Age Group Mean (Standard Error) Characteristics of Pill Burden, Adherence, and PMMQ Strategies Endorsed (i.e., at least Sometimes) in a Strategy Scale
| Variable | Older HIV Infected (n = 117) |
Younger HIV Infected (n = 82) |
|---|---|---|
| Pill Burden | ||
| ARV per day (# of doses) | 4.5 (0.3) | 4.0 (0.3) |
| ARV Burden (% above median) | 42.7 | 36.6 |
| Non-ARV per day (# of non-ARV medications)* | 5.3 (0.4)* | 2.9 (0.4)* |
| Non-ARV Burden (% above median)* | 65.8* | 43.9* |
| Total Medications* | 8.9 (0.4)* | 6.2 (0.4)* |
| Total Medications Burden (% above median)* | 55.6* | 28.1* |
| Adherence | ||
| ARV Adherent at 90% correct doses (%) | 50.40 | 51.20 |
| ARV Mean Adherence (% correct doses taken) | 85.23 (2.1) | 88.0 (2.0) |
| Number of Days Monitored (MEMS) | 39.4 (0.7) | 39.2 (1.0) |
| PMMQ Strategies | ||
| Total Strategies (of 112)* | 32.3 (1.6)* | 24.7 (1.7)* |
| External | ||
| Prospective (of 28)* | 5.4 (0.4)* | 3.8 (0.4)* |
| Retrospective (of 28)* | 4.2 (0.4)* | 2.6 (0.3)* |
| Internal | ||
| Prospective (of 16) | 6.8 (0.3) | 6.0 (0.4) |
| Retrospective (of 40)* | 15.8 (0.8)* | 12.2 (1.0)* |
Note. ART = Antiretroviral therapy; PMMQ = Prospective Memory for Medications Questionnaire; MEMS = Medication Event Monitoring System;
p < .05.
Neurobehavioral Assessment
PM assessment
We assessed PM with a standardized, performance-based measure administered in the laboratory. We administered the Memory for Intentions Screening Test (MIST), which has been shown to have high reliability (subscale Cronbach’s α = .886; Woods, Moran, Dawson, et al., 2008) and validity (Carey, Woods, Rippeth, et al., 2004). During the administration of the MIST, the participant completed an ongoing word search distractor task and was asked to complete eight total PM actions at specified times or event-based cues as directed by the experimenter. The eight PM trials were comprised of four time-based (e.g., In 15 minutes, tell me that it is time to take a break) and four event-based (e.g., When I show you a postcard, self-address it) cues. There are two possible points for each MIST trial: one for responding at the correct time (i.e., within 15% of the target time for time-based trials) or to the appropriate cue (for event-based trials) and one for a correct response. These trials are then summed across cue type to create time-based and event-based subscales (ranges = 0 - 8).
ARV adherence
Adherence classifications were determined based on the outcome of 4 weeks of continuous tracking utilizing the non-alarm Medication Event Monitoring System (MEMS; Aprex Corporation, Union City, CA), which began on the day following evaluation. The MEMS recorded the date and time the participants opened the medication bottle over the 4-week period. We selected a “sentinel” ARV to be tracked via MEMS. Because protease inhibitor (PI) medications are most sensitive to non-adherence, we selected these medications for tracking if the participant was prescribed a PI. For individuals not prescribed a PI, we selected as the sentinel medication the participant’s nucleoside reverse transcriptase inhibitor or non-nucleoside reverse transcriptase inhibitor that was dosed most frequently, or any fixed-dose combination tablet (e.g., tenofovir/emtricitabine) if a participant was prescribed one. Percent adherence was calculated as the proportion of correct bottle openings over the 30-day period (i.e., [(Number of recorded bottle openings)/(Number of prescribed doses)]*100%). Participants then were classified as adherent or non-adherent based on a 90% cut-point, as is consistent with the literature demonstrating the optimal level of adherence as 95-100% for viral suppression (Bangsberg, 2008) and increased risk of poorer outcomes below 90% adherence (Hinkin et al., 2002).
ARV adherence strategies
The Prospective Memory for Medications Questionnaire (PMMQ) was administered to assess utilization of 28 memory strategies that aid medication adherence. On this measure, participants reported whether they used each strategy never (0), seldom (1), sometimes (2), often (3), or always (4), and whether any item used was effective (e.g., highly ineffective [3] to highly effective [0]). Items were categorized into four possible types of adherence strategy based on whether or not the strategy was (a) external vs. internal (e.g., using a pillbox vs. repeatedly thinking about having to take the medication and (b) retrospective vs. prospective (e.g., remembering whether you’ve taken medication vs. a reminder to take medication). Thus, four possible strategies (external retrospective, external prospective, internal retrospective, internal prospective) included: external strategies for retrospective aspects of adherence (7 items; e.g., Do you use a dated pillbox to help you make sure you take the right amount of medication per day?); external strategies for prospective aspects of adherence (7 items; e.g., Do you use a clock or watch alarm to remind you when it is time to take your medication?); internal strategies for retrospective aspects of adherence (10 items; e.g., Do you regularly repeat to yourself the instructions for taking a prescription that you’ve been taking for a long time?); and internal strategies for prospective aspects of adherence (4 items; e.g., At the beginning of the day, do you think about when you need to take your medication so you can include your medication into your day’s schedule?). If participants were missing one item on a given strategy scale, the individual’s mean score for the scale was imputed for that item; if more than one item was missing on a strategy scale, the participant had a missing score for that scale. Scores were determined by adding all of the items together (total possible range for frequency of strategy use = 0 - 112; higher scores indicated more frequent strategy use). For ratings of effectiveness, each strategy category was considered to be self-rated as effective if a participant endorsed using at least one strategy at least sometimes in that category and rated the same strategy as effective (i.e., 1 = effective or 0 = highly effective).
Medication Management Self-efficacy
All participants completed the 20-item Medication Management Efficacy Scale (MMES; e.g., I am less efficient at adhering to my medication regimen than I used to be) questionnaire of the Beliefs Related to Medication Adherence (BERMA). All items on the MMES were self-rated from 5 = strongly agree to 1 = strongly disagree), with higher values reflecting better self-rated efficacy of medication management.
Neuropsychological Evaluation
Participants were administered a standardized neuropsychological battery by trained research assistants. The battery assessed key domains outlined in the Frascati criteria for HAND (Antinori et al., 2007), including motor skills, learning, memory, attention, executive functions, and processing speed (as described in Woods et al., 2013). Using normative demographic data, raw neuropsychological test scores were transformed into T-scores, which were subsequently translated into deficit scores that ranged from 0 = normal to 5 = severe (Carey, Woods, Gonzalez, et al., 2004). Deficit scores were then averaged to generate a global deficit score (GDS; Carey, Woods, Gonzalez, et al., 2004) wherein higher values reflect greater levels of neurocognitive impairment.
Psychiatric interview
In order to characterize the HIV-infected age groups (see Table 1) and to determine possible covariates that might be related to PM, adherence, or the HIV-infected age groups, we diagnosed lifetime and current (within 1 month prior to evaluation) Substance-Related Disorders, Generalized Anxiety Disorder, and Major Depressive Disorder; all participants were administered the Composite International Diagnostic Interview (version 2.1).
Statistical Analyses
First, we performed identical logistic regressions in each age group (i.e., older and younger) predicting the outcome variable MEMS-based adherence status (i.e., adherent or non-adherent), with MIST time-based or event-based subscale (conducted separately), total medication pill burden, and the interaction as predictors of interest. All variables in Table 1 were examined as possible covariates, with no variables showing significant associations with both age group and the outcome of adherence. However, given that lifetime history of depression is a known predictor of adherence, we included any lifetime diagnosis of Major Depressive Disorder in the model as a covariate. Given the non-normal distribution of the MIST time- and event-based subscales, each of these logistic regressions was then followed by non-parametric (i.e., Wilcoxon Rank-Sum Tests) pairwise comparison post-hocs to explore both significant univariate and interaction terms. (Note: Similar analyses were also performed with non-ARV and ARV burden, as well as with parametric follow-up regressions, but primary findings remained consistent with what is reported below).
Results
PM, Pill Burden, and Adherence
Time-based MIST
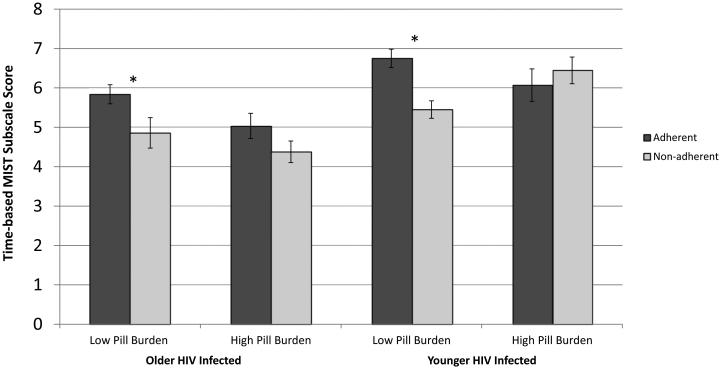
In the older group, the overall model that included time-based MIST, overall pill burden, and their interaction predicting adherence was significant, χ2 (4) = 12.57, p = 0.014. Better performance on time-based MIST was associated with higher likelihood of adherence in the older group, χ2 (1) = 8.21, p = 0.004, d = 0.55, irrespective of ARV pill burden (univariate and interaction terms ps > 0.1). Within the younger group, the overall model also was significant, χ2 (4) = 17.24, p = 0.002, and showed a significant interaction between time-based MIST and pill burden, χ2 (1) = 6.63, p = 0.01, but the main effects for pill burden and time-based MIST did not reach significance (ps > .10). As shown in Figure 1, follow-up analyses demonstrated that better time-based MIST performance was related to a higher likelihood of adherence only in participants with low total pill burden (χ2 [1] = 13.85, p < .001, d = 1.05v), but time-based MIST was not related to adherence in the high pill burden group (χ2 [1] = 0.238, p = 0.626, d = 0.29). In the older group, adherence rates were 59.6% in the low burden group and 43.1% in the high burden group. In the younger group, adherence rates were 47.5% in the low burden group and 60.9% in the high burden group.
Figure 1.
Prospective memory scores and medication adherence within high and low ART load groups in older and younger HIV-infected adults.
Note. MIST = Memory for Intentions Screening Test, *p < .05.
Event-based MIST
Both logistic regressions in the older and younger HIV samples failed to show significant effects of event-based MIST on adherence (all ps > 0.10). Additionally, there was no interaction with pill burden in either age group (ps > 0.10). To confirm these results, we conducted pair-wise post-hoc comparisons of event-based MIST scores predicting performance—both across and within levels of pill burden—which did not reach significance for any comparison (all ps > .10).
Post-hoc Analyses in the Younger Group
Given the unexpected significant interaction between time-based MIST and pill burden in the younger group, we conducted a series of post-hoc one-way analyses of variance (ANOVA; or Wilcoxon Rank-Sum non-parametric tests for non-normally distributed data) comparing the younger high- and low-pill-burden groups on several possible explanatory factors, including medication strategy use and effectiveness (i.e., PMMQ), medication management self-efficacy (i.e., BERMA), HIV disease severity (e.g., nadir CD4+ T cell count, AIDS diagnosis), and global neurocognitive functioning (i.e., GDS).
Medication strategy use and effectiveness
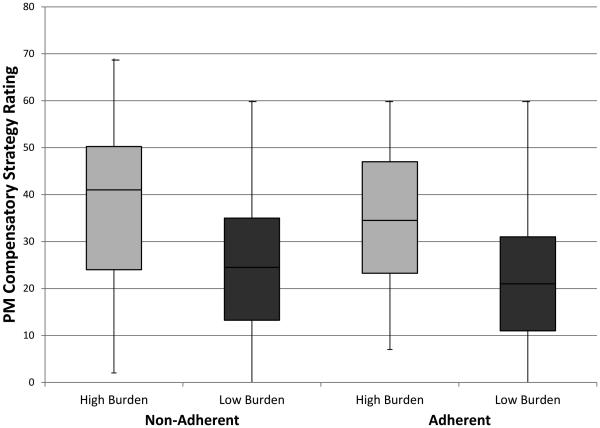
Figure 2 shows the number of total strategies endorsed as used at least sometimes by the younger HIV-infected group on a stand-alone self-report of prospective memory compensatory strategies. Within the younger group, individuals with high pill burden rated using PMMQ total strategies more frequently than their low pill burden counterparts (F[1,80] = 11.48, p = .001, d = .81; see Figure 2), regardless of whether adherent or non-adherent. Younger individuals with high pill burden also had a significantly higher proportion of individuals who used external prospective strategies and rated them as effective (Fischer’s exact test p = .041; odds ratio = 3.49, 95% CI [1.06, 11.54]) compared to individuals with low pill burden.
Figure 2.
Boxplots for total memory strategy frequency of use on the Prospective Memory for Medications Questionnaire in low and high total pill burden groups for non-adherent and adherent younger HIV-infected adults.
Note. PM = prospective memory.
Medication Management Self-Efficacy
In the younger group, individuals with high pill burden had significantly lower ratings of medication management self-efficacy on the BERMA, F(1,77) = 4.17, p = .045, d = .52, compared to the low pill burden group.
Disease severity
In the younger group, individuals with high pill burden were significantly more likely to have an AIDS diagnosis (Fischer’s exact test p = .013; odds ratio = 4.29, 95% CI [1.30, 14.15]) and longer estimated durations of infection (χ2 [1]= 4.80, p =.029, d = 0.57) compared to younger individuals with low pill burden. There were no significant differences between the pill burden groups on the likelihood of having detectable HIV RNA in plasma or cerebrospinal fluid, or current and nadir CD4+ T cell counts (ps > .10).
Global cognitive functioning
Within the younger group, individuals with high pill burden did not differ on GDS scores compared to the low pill burden group, χ2 [1]= 1.83, p = .176.
Discussion
Our study was guided by the hypothesis that older HIV-infected adults with time-based PM impairment may be at greater risk of ARV non-adherence, particularly in the setting of higher pill burden. As is often the case in clinical science, our results suggested a somewhat different pattern of findings than what was expected based on our literature review. In older HIV-infected adults, lower time-based MIST scores were significantly associated with worse ARV adherence, yet this relationship did not vary by level of pill burden. In younger HIV-infected adults, however, lower time-based MIST scores were associated with worse ARV adherence only in the setting of lower pill burden. This surprising association had a very large effect size (d = 1.05) and was not confounded by other demographic or clinical factors (i.e., lifetime diagnosis of Major Depressive Disorder). As such, the results of our study indicate that for younger HIV-infected individuals, those with lower pill burdens may be at disproportionate risk for ARV non-adherence in the context of time-based MIST impairment. The potential explanations for this intriguing finding and its clinical implications are discussed below.
One possibility suggested by our data is that the association between the time-based MIST and medication adherence in younger HIV-infected adults might be dependent on the level of strategic environmental demands conferred by pill burden. Time-based MIST performance was related to adherence only in the low pill burden group (see Figure 1), which was unexpected given the considerable body of evidence suggesting that medication adherence would be improved with lower pill burden (Buscher, Hartman, Kallen, & Giordano, 2012; Maggiolo et al., 2002; O’Connor et al., 2013). However, further exploration of medication management strategies used by younger HIV-infected adults revealed that individuals with lower pill burdens endorsed less frequent use of PM strategies (e.g., using a clock or watch alarm to indicate when it was time to take medications; see Figure 2). Thus, some individuals (e.g., those with poor time-based PM) within this group may be underutilizing compensatory strategies that could buffer the adverse affects of impaired PM capacity. While the mechanisms driving this finding occurring only in the low pill burden group were likely multifaceted, it is possible that younger HIV-infected adults with low pill burden may not feel at risk for medication non-adherence. Indeed, they reported slightly greater medication management self-efficacy (as measured by the BERMA). Thus, it may be helpful to target this group with interventions and compensatory strategies as needed.
Another (not mutually exclusive) possibility explaining the differences in the association between time-based MIST and adherence between the pill burden groups may be factors in the younger high burden group that resulted in this group overcoming the normal effects of time-based PM on adherence. Post-hoc analyses revealed that for younger adults, individuals with high pill burdens had more severe HIV disease (i.e., AIDS, durations of infection), poorer medication management self-efficacy, and employed more memory strategies as compared to younger adults with low pill burden. Taken together, the younger group with high pill burden may have had greater awareness of their illness (e.g., higher disease severities, lower medication management self-efficacy) and recognition of needing adherence strategies (e.g., higher PMMQ strategy frequency), which might have helped them overcome the commonly observed adverse effects of time-based PM on adherence. Interestingly, the younger HIV-infected group did not differ in the number of prescribed ARV medications compared to older adults (Note: data showed that the higher pill burden group did have more advanced disease, which may have required more aggressive therapy), and this unexpected effect in the age of single fixed dose tablets was a limitation of our samples, as patients continued to be prescribed lower ARV burdens. While it is important to note that the high and low pill burden groups in the younger HIV-infected group did not differ on the outcome of ARV adherence (see Figure 2), our study highlights a group in younger adults who may overcome the typical time-based PM-related barriers to ARV adherence.
Pill burden did not affect the strong association between time-based MIST and ARV non-adherence for the older adults. This finding contrasted with previous studies indicating that neurocognitive impairment was more closely associated with medication non-adherence in older age (Hinkin et al., 2004). One possible explanation for this finding was the way in which this group implemented compensatory strategies in the real world. While older and younger adults did not differ in the frequency of using compensatory strategies (post hoc analyses revealed this effect was true regardless of pill burden; high pill burden age groups: F[1,86] = 1.09, p = .300, d = .36; low pill burden age groups: F(1,109) = 2.84, p = .01, d = .32), it was possible that the older adults integrated these strategies more effectively (e.g., better integrated into more structured daily routines). On the PMMQ ratings of effectiveness, we found that only younger adults with low pill burden had a lower proportion of individuals that used external prospective strategies and rated them as effective compared to older HIV-infected adults (Fischer’s exact test p = .005; odds ratio = 2.71, 95% CI [1.37, 5.34]). Indeed, findings of an “age-PM paradox” in the literature suggested that, while older adults were at a higher risk for PM failures in laboratory-based tasks, this effect was attenuated (or even reversed) such that older and younger HIV-infected adults performed comparably on naturalistic PM tasks in the real world (Weber et al., 2011). However, our finding that adherence remained stable across these groups precludes any meaningful results to be drawn from our data. Still, it is possible that lifestyle differences between older and younger adults (e.g., employment, motivation) impacted the number of compensatory strategies that the younger adults, especially those with low pill burden, may have employed. Future research is needed to address whether there are age-related differences in the effectiveness (e.g., salience of external memory aids) of self-reported compensatory strategies on resulting ARV adherence. Regardless, this finding may highlight a group within the greater HIV population that could benefit from medication management training programs, such as spaced retrieval interventions (Neundorfer et al., 2004). For example, patients might be prompted to recall the content of the intention to be remembered (i.e., taking medication) when cued, with correct performance yielding increasingly longer duration between testing cues, until the patient is able to correctly recall the intention without error for 3 consecutive testing sessions.
The findings of our study underscore the potential importance of time-based PM for effectively adhering to ARVs in the context of HIV, especially in younger individuals who do not choose or are unable to implement external PM compensatory strategies. These data support previous findings that time-based PM plays a critical role in ARV adherence (Woods et al., 2009). In parallel, a recent study in older adults without HIV infection by Woods et al. (2014) found that the strategic aspects of event-based PM, which involved using a cue that was not semantically related to the intended action (e.g., taking a medication before bed that was supposed to be paired with a meal), played a critical role in medication adherence. In the context of the present study, our findings highlighted not only the importance of time-based PM performance in ARV adherence, but also underscored how ancillary executive processing of strategic demands due to pill burden may affect overall adherence in the context of time-based PM. For instance, the strategic demands placed on time-based PM may be higher in the context of lower pill burden due to increased length of monitoring intervals between taking pills, thus resulting in our observed findings in younger adults.
Finally, while increased attention has been paid to potential compensatory training strategies as they relate to PM and medication adherence across the lifespan or later in life (Zogg et al., 2012), no studies to our knowledge have examined these possible interventions explicitly for younger adults. In parallel, there is a general agreement in the literature suggesting that lower pill burden results in improved adherence (Maggiolo et al., 2002; O’Connor et al., 2013). However, the present data suggest that there may be nuances in the interactions between specific cognitive processes that are often utilized in medication management programs (i.e., PM) and the ARV regimens prescribed to individuals infected with HIV. As such, these data suggest that those in clinical nursing practice may want to consider pill burden as well as age group when determining the possible effects of PM on adherence. These findings are especially relevant to nurses in care settings that include both younger and older HIV-infected individuals, as the effects of pill burden on the association between PM performance and adherence may be expected to differ in these groups. Furthermore, our study highlights the need to study the potential consequences of poor medication adherence in low ARV pill burden groups, for whom a missed dose may result in missing a higher proportion of the daily ARV regimen. Our findings highlight the need for empirical questions to be investigated with regard to interventions aimed at improving adherence across the lifespan. Interventions in clinical practice aimed at ameliorating aging-related PM deficits may be insufficient to address the needs of younger HIV-infected populations that may be undergoing psychosocial stressors not experienced as often later in life, such as occupational time management or social pressure.
Key Considerations.
Among younger adults infected with HIV, impairment on time-based prospective memory was related to non-adherence only in persons with lower pill burden.
Younger HIV-infected individuals with higher pill burden may overcome the adverse effects of time-based prospective memory on adherence by using compensatory medication taking strategies (e.g., pillboxes).
Health care professionals in clinical practice may want to consider pill burden as well as the age group being treated when determining the possible effects of prospective memory deficits on adherence.
Interventions aimed at ameliorating prospective memory deficits and their effects on adherence need to be tailored across the lifespan.
Acknowledgments
This work was supported by the National Institutes of Health under Grants R01-MH073419, T32-DA31098, L30-DA0321202, P30-MH62512 and K24-AG026431. The San Diego HIV Neurobehavioral Research Program [HNRP] group is affiliated with the University of California, San Diego; the Naval Hospital, San Diego; and the Veterans Affairs San Diego Healthcare System; including Director, Igor Grant, MD; Co-Directors, J. Hampton Atkinson, MD; Ronald J. Ellis, MD, PhD; and J. Allen McCutchan, MD; Center Manager, Thomas D. Marcotte, PhD; Jennifer Marquie-Beck, MPH; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, MD, PhD (PI); J. Allen McCutchan, MD; Scott Letendre, MD; Edmund Capparelli, PharmD; Rachel Schrier, PhD; Debra Rosario, MPH; Neurobehavioral Component: Robert K. Heaton, PhD (PI); Mariana Cherner, PhD; Jennifer E. Iudicello, PhD; David J. Moore, PhD; Erin E. Morgan, PhD; Matthew Dawson; Neuroimaging Component: Terry Jernigan, PhD (PI); Christine Fennema-Notestine, PhD; Sarah L. Archibald, MA; John Hesselink, MD; Jacopo Annese, PhD; Michael J. Taylor, PhD; Neurobiology Component: Eliezer Masliah, MD (PI); Cristian Achim, MD, PhD; Ian Everall, FRCPsych, FRCPath, PhD (Consultant); Neurovirology Component: Douglas Richman, MD (PI); David M. Smith, MD; International Component: J. Allen McCutchan, MD (PI); Developmental Component: Cristian Achim, MD, PhD (PI); Stuart Lipton, MD, PhD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI); Data Management Unit: Anthony C. Gamst, PhD (PI), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, PhD (PI); Florin Vaida, PhD; Reena Deutsch, PhD; Anya Umlauf, MS. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank Marizela Cameron and P. Katie Riggs for their help with study management and Donald Franklin and Stephanie Corkran for their help with data processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Contributor Information
David P. Sheppard, University of Houston, Houston, Texas, USA.
Erica Weber, Kessler Foundation, West Orange, New Jersey, USA.
Kaitlin B. Casaletto, University of California, San Francisco, San Francisco, California, USA.
Gunes Avci, University of Houston, Houston, Texas, USA.
Steven Paul Woods, University of Houston, Houston, Texas, USA.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. doi:10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. Journal of Infectious Diseases. 2008;197(3):272–278. doi: 10.1086/533415. doi:10.1086/533415. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Woods SP, Weber E, Grant I, Moore DJ. Memory-based strategies for antiretroviral medication management: An evaluation of clinical predictors, adherence behavior awareness, and effectiveness. AIDS and Behavior. 2013;17(1):74–85. doi: 10.1007/s10461-012-0308-9. doi:10.1007/s10461-012-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscher A, Hartman C, Kallen MA, Giordano TP. Impact of antiretroviral dosing frequency and pill burden on adherence among newly diagnosed, antiretroviral-naive HIV patients. International Journal of STD & AIDS. 2012;23(5):351–355. doi: 10.1258/ijsa.2011.011292. doi:10.1258/ijsa.2011.011292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26(3):307–319. doi: 10.1080/13803390490510031. doi:10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Heaton RK. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clinical Neuropsychologist. 2004;18(2):234–248. doi: 10.1080/13854040490501448. doi:10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention HIV Surveillance Report, 2013. 2015 Retrieved from http://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-vol-25.pdf.
- Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, Lam M, Foley J. Aging, neurocognition, and medication adherence in HIV infection. American Journal of Geriatric Psychiatry. 2009;17(4):281–290. doi: 10.1097/JGP.0b013e31819431bd. doi:10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TR, Sterne JAC, Schneider M-P, De Geest S, Nicca D, Furrer H, Bucher HC. Self-reported nonadherence to antiretroviral therapy as a predictor of viral failure and mortality. AIDS. 2015;29(16):2195–2200. doi: 10.1097/QAD.0000000000000782. doi:10.1097/QAD.0000000000000782. [DOI] [PubMed] [Google Scholar]
- Gupta S, Woods SP, Weber E, Dawson MS, Grant I. Is prospective memory a dissociable cognitive function in HIV infection? Journal of Clinical and Experimental Neuropsychology. 2010;32(8):898–908. doi: 10.1080/13803391003596470. doi:10.1080/13803391003596470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Stefaniak M. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. doi:10.1212/WNL.61.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(1):19–25. doi: 10.1097/00002030-200418001-00004. doi:10.1111/j.1460-9568.2012.08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks A, Wainwright DA, Salazar L, Grimes R, York M, Strutt AM, Hasbun R. Neurocognitive deficits increase risk of poor retention in care among older adults with newly diagnosed HIV infection. AIDS. 2015;29(13):1711–1714. doi: 10.1097/QAD.0000000000000700. doi:10.1097/QAD.0000000000000700. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Cain D, Cherry C, Kalichman M, Pope H. Pillboxes and antiretroviral adherence: prevalence of use, perceived benefits, and implications for electronic medication monitoring devices. AIDS Patient Care and STDs. 2005;19(12):833–839. doi: 10.1089/apc.2005.19.833. doi:10.1089/apc.2005.19.833. [DOI] [PubMed] [Google Scholar]
- Krentz HB, Cosman I, Lee K, Ming JM, Gill MJ. Pill burden in HIV infection: 20 years of experience. Antiviral Therapy. 2012;17(5):833–840. doi: 10.3851/IMP2076. doi:10.3851/IMP2076. [DOI] [PubMed] [Google Scholar]
- Lee DS, De Rekeneire N, Hanlon JT, Gill TM, Bauer DC, Meibohm B, Jeffery SM. Cognitive impairment and medication complexity in community-living older adults: The health, aging and body composition study. Journal of Pharmacy Technology. 2012;28(4):156–162. doi: 10.1177/875512251202800405. doi:10.1177/875512251202800405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Gallien S, Ribaudo H, Heisey A, Bangsberg DR, Kuritzkes DR. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS. 2014;28(2):181–186. doi: 10.1097/QAD.0000000000000123. doi:10.1097/QAD.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, Weinborn M, Loft S, Maybery M. Patterns of prospective memory impairment among individuals with depression: The influence of cue type and delay interval. Journal of the International Neuropsychological Society. 2013;19(6):718–722. doi: 10.1017/S1355617713000180. doi:10.1017/S1355617713000180. [DOI] [PubMed] [Google Scholar]
- Liu AY, Hessol NA, Vittinghoff E, Amico KR, Kroboth E, Fuchs J, Buchbinder SP. Medication adherence among men who have sex with men at risk for HIV infection in the United States: Implications for pre-exposure prophylaxis implementation. AIDS Patient Care and STDs. 2014;28(12):622–627. doi: 10.1089/apc.2014.0195. doi:10.1089/apc.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolo F, Ripamonti D, Arici C, Gregis G, Quinzan G, Camacho GA, Suter F. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clinical Trials. 2002;3(5):371–378. doi: 10.1310/98b3-pwg8-pmyw-w5bp. doi:10.1310/98B3-PWG8-PMYW-W5BP. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA. Antiretroviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS. 2012;26(1):39–53. doi: 10.1097/QAD.0b013e32835584ea. doi:10.1097/QAD.0b013e32835584ea. [DOI] [PubMed] [Google Scholar]
- Neundorfer MM, Camp CJ, Lee MM, Skrajner MJ, Malone ML, Carr JR. Compensating for cognitive deficits in persons aged 50 and over with HIV/AIDS: A pilot study of a cognitive intervention. Journal of HIV/AIDS & Social Services. 2004;3(1):79–97. doi:10.1300/J187v03n01_07. [Google Scholar]
- O’Connor JL, Gardner EM, Mannheimer SB, Lifson AR, Esser S, Telzak EE, Phillips AN. Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. Journal of Infectious Diseases. 2013;208(1):40–49. doi: 10.1093/infdis/jis731. doi:10.1093/infdis/jis731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DE, Woods SP, Franklin D, Cattie JE, Heaton RK, Collier AC, Grant I. Relationship of Medication Management Test-Revised (MMT-R) performance to neuropsychological functioning and antiretroviral adherence in adults with HIV. AIDS and Behavior. 2012;16(8):2286–2296. doi: 10.1007/s10461-012-0237-7. doi:10.1007/s10461-012-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poquette AJ, Moore DJ, Gouaux B, Morgan EE, Grant I, Woods SP. Prospective memory and antiretroviral medication non-adherence in HIV: An analysis of ongoing task delay length using the memory for intentions screening test. Journal of the International Neuropsychological Society. 2013;19(2):155–161. doi: 10.1017/S1355617712001051. doi:10.1017/S1355617712001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr L, Lampe FC, Clucas C, Johnson M, Fisher M, Leake Date H, Harding R. Self-reported non-adherence to ART and virological outcome in a multiclinic UK study. AIDS Care. 2010;22(8):939–945. doi: 10.1080/09540121.2010.482126. doi:10.1080/09540121.2010.482126. [DOI] [PubMed] [Google Scholar]
- Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A, Hallett TB. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet, Infectious Diseases. 2015;15(7):810–818. doi: 10.1016/S1473-3099(15)00056-0. doi:10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, Hinkin CH. Medication and finance management among HIV-infected adults: The impact of age and cognition. Journal of Clinical and Experimental Neuropsychology. 2011;33(2):200–209. doi: 10.1080/13803395.2010.499357. doi:10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Gakumo CA. The impact of neuropsychological performance on everyday functioning between older and younger adults with and without HIV. Journal of the Association of Nurses in AIDS Care. 2013;24(2):112–125. doi: 10.1016/j.jana.2012.05.002. doi:10.1016/j.jana.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Woods SP, Delano-Wood L, Bondi MW, Gilbert, Grant I. An examination of the age-prospective memory paradox in HIV-infected adults. Journal of Clinical and Experimental Neuropsychology. 2011;33(10):1108–1118. doi: 10.1080/13803395.2011.604027. P. E. doi:10.1080/13803395.2011.604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH. Timing is everything: Antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15(1):42–52. doi: 10.1017/S1355617708090012. doi:10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2010;32(4):398–407. doi: 10.1080/13803390903130737. doi:10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Hoebel C, Pirogovsky E, Rooney A, Cameron MV, Grant I, Gilbert PS. Visuospatial temporal order memory deficits in older adults with HIV infection. Cognitive and Behavioral Neurology. 2013;26(4):171–180. doi: 10.1097/WNN.0000000000000013. doi:10.1097/WNN.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, Atkinson JH. Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology. 2008;23(3):257–270. doi: 10.1016/j.acn.2007.12.006. doi:10.1016/j.acn.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I. Psychometric characteristics of the memory for intentions screening test. Clinical Neuropsychologist. 2008;22(5):864–878. doi: 10.1080/13854040701595999. doi:10.1080/13854040701595999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre SL. Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cognitive and Behavioral Neurology. 2006;19(4):217–221. doi: 10.1097/01.wnn.0000213916.10514.57. doi:10.1097/01.wnn.0000213916.10514.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Maxwell BR, Gummery A, Mo K, Ng ARJ, Bucks RS. Event-based prospective memory is independently associated with self-report of medication management in older adults. Aging & Mental Health. 2014;18(6):745–753. doi: 10.1080/13607863.2013.875126. doi:10.1080/13607863.2013.875126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Martin K, Corbett A, Napravnik S, Eron J, Zhu Y, Wohl DA. Total daily pill burden in HIV-infected patients in the southern United States. AIDS Patient Care and STDs. 2014;28(6):311–317. doi: 10.1089/apc.2014.0010. doi:10.1089/apc.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg JB, Woods SP, Sauceda JA, Wiebe JS, Simoni JM. The role of prospective memory in medication adherence: A review of an emerging literature. Journal of Behavioral Medicine. 2012;35(1):47–62. doi: 10.1007/s10865-011-9341-9. doi:10.1007/s10865-011-9341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]