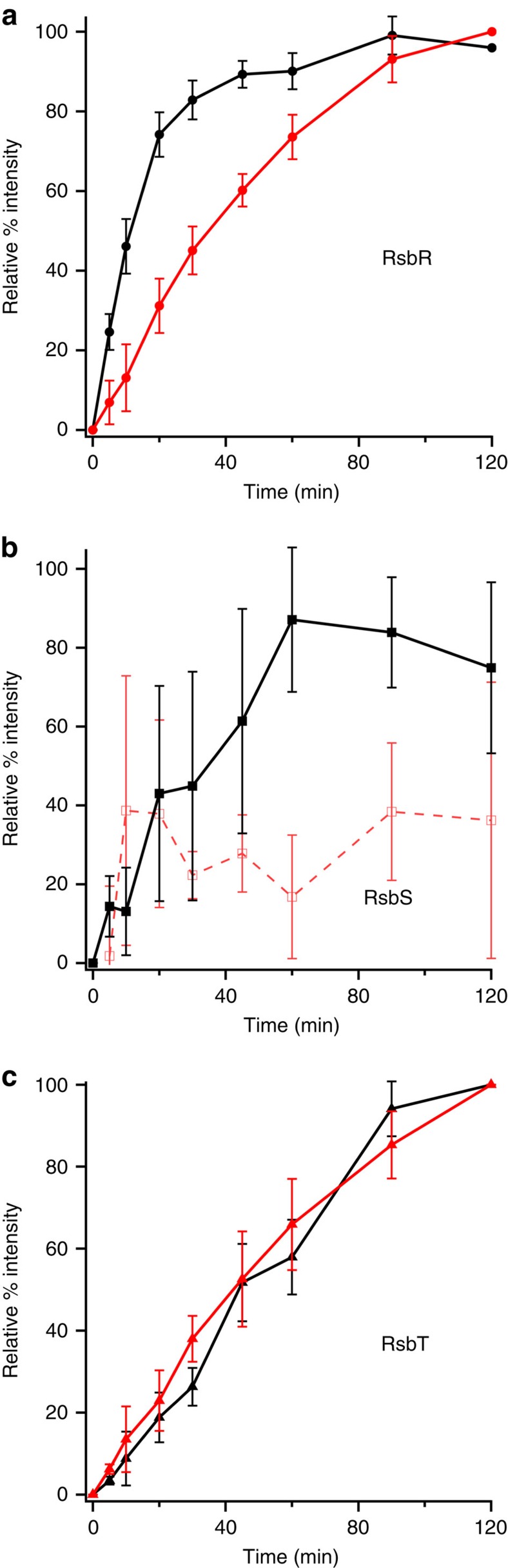
Figure 3. Phosphorylation kinetics of kinase RsbT in the presence of RsbR in various ligation states and RsbS.
Average rate of phosphorylation of RsbR (a) RsbS (b) and RsbT (c) in a RsbR/S/T mixture (ligation/oxidation states of RsbR: FeII-O2, red; FeII unligated, black). All experiments were performed at least in triplicate, and mean and s.d. values are plotted for each time point. Phosphorylation of RsbS was not detected above background levels when FeII-O2 RsbR was included in the reaction mixture and therefore did not show any time-dependent kinetics (variability in the RsbS FeII-O2 plots is due to normalization within the background signals).