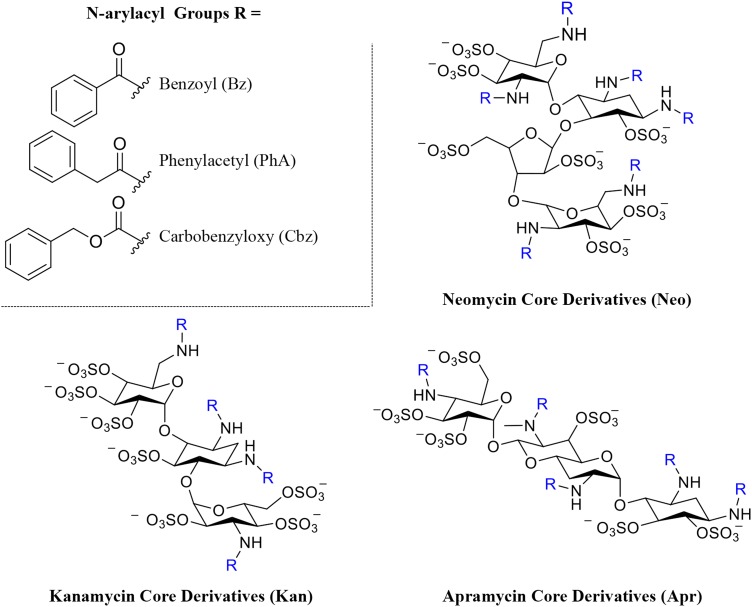
Fig. 1.
Structures and degree of sulfation (DS) of N-arylacyl O-sulfonated aminoglycosides used in this study. The three aminoglycoside neomycin, kanamycin and apramycin are N-substituted with three different arylacyl groups and per-O-sulfonated, affording the panel of nine derivatives: N-carbobenzyloxy O-sulfonated neomycin (NeoCbz, DS = 7), N-phenylacetyl O-sulfonated neomycin (NeoPhA, DS = 6.8), N-benzoyl O-sulfonated neomycin (NeoBz, DS = 7), N-carbobenzyloxy O-sulfonated kanamycin (KanCbz, DS = 6), N-phenylacetyl O-sulfonated kanamycin (KanPhA, DS = 5.8), N-benzoyl O-sulfonated kanamycin (KanBz, DS = 5.1), N-carbobenzyloxy O-sulfonated apramycin (AprCbz, DS = 6), N-phenylacetyl O-sulfonated apramycin (AprPhA, DS = 6), N-benzoyl O-sulfonated apramycin (AprBz, DS = 6). Complete synthesis and characterization of panel of compounds is described in Supplementary data. This figure is available in black and white in print and in color at Glycobiology online.