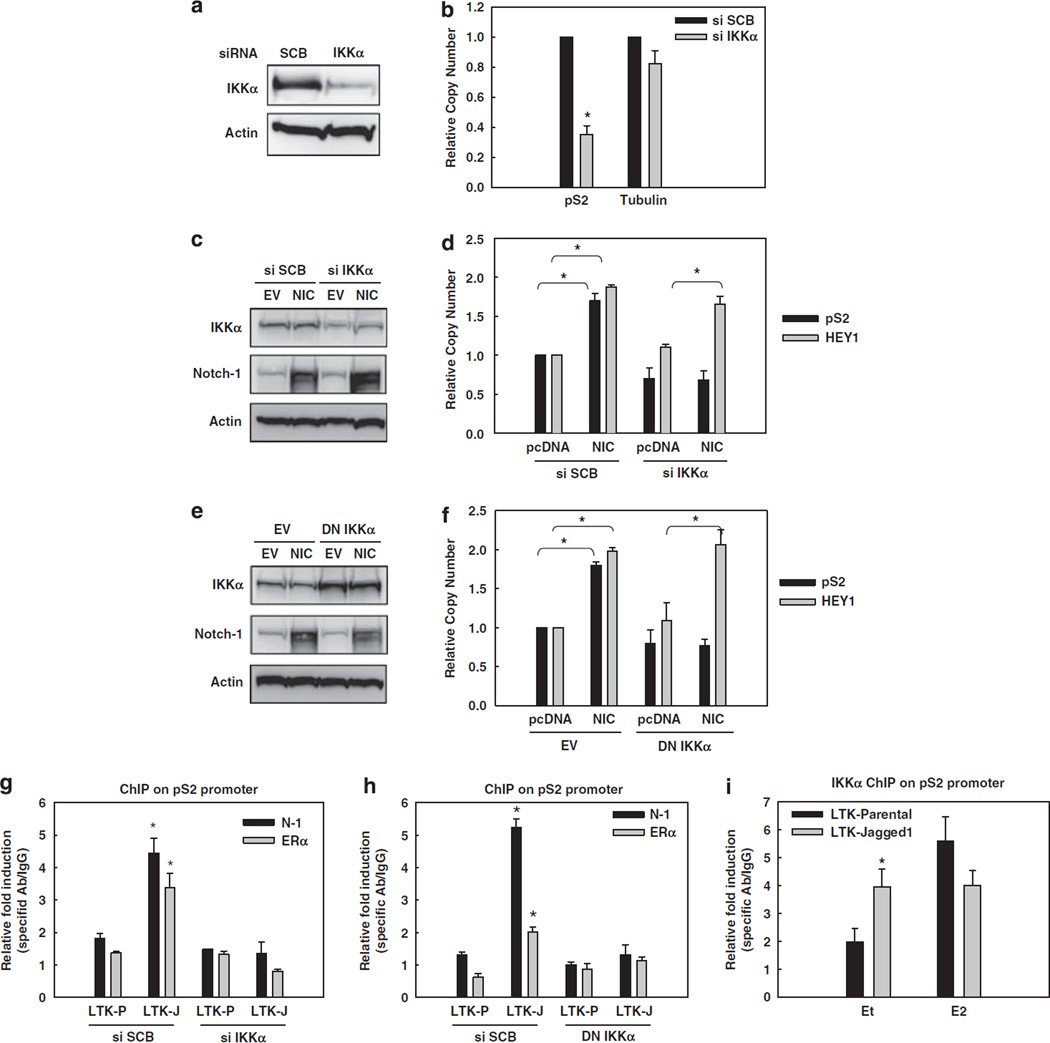
Figure 3.
Notch-1 requires IKKα and its kinase activity for the transcriptional activation of ER-dependent genes. In all experiments, MCF-7 cells were grown in charcoal-stripped medium for a total of 3 days. (a) MCF-7 cells were transfected with IKKα siRNA. The expression level of IKKα was measured by western blotting. (b) pS2 real-time RT–PCR was performed with the same cells as in panel a. Data are expressed as relative copy number after normalization to internal control 18 S rRNA. Tubulin was used as negative control; *P < 0.0001. (c) MCF-7 cells were co-transfected with IKKα siRNA or scrambled control and the construct expressing NIC or pcDNA control. Overexpression of NIC and downregulation of IKKα were validated by western blotting, using actin as loading control. (d) HEY1 and pS2 real-time RT–PCR were performed with the same cells as in panel c; * P ⩽ 0.001. (e) MCF-7 cells were co-transfected with DN-IKKα (AA) or the empty vector and NIC or pcDNA control. Overexpression of DN-IKKα (AA) and Notch-1 was validated by western blotting, using actin as loading control. (f) HEY1 and pS2 real-time RT–PCR were performed with the same cells as in panel e; *P ⩽ 0.001. (g, h) MCF-7 cells were transfected with IKKα siRNA (g) or DN-IKKα (h) and co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h. ChIP assays were performed with antibodies to Notch-1 or ERα, followed by real-time PCR analysis of the pS2 promoter; *P ⩽ 0.001. (i) MCF-7 cells were co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h and treated with 5 nm E2 or ethanol control for 1 h prior to harvest. ChIP assay was performed with IKKα antibody followed by real-time PCR for the pS2 promoter; *P ⩽ 0.001. ChIP, chromatin immunoprecipitation; DN-IKKα, the dominant-negative form of IKKα; ERα, estrogen receptor-α; GSI, γ-secretase inhibitor; LTK–JAG1, mouse LTK fibroblasts expressing Notched ligand Jagged-1; LTK–PAR, control vector-transduced parental fibroblast; NIC, active form of Notch-1 (Notch-1IC); RT–PCR, reverse transcription–PCR.