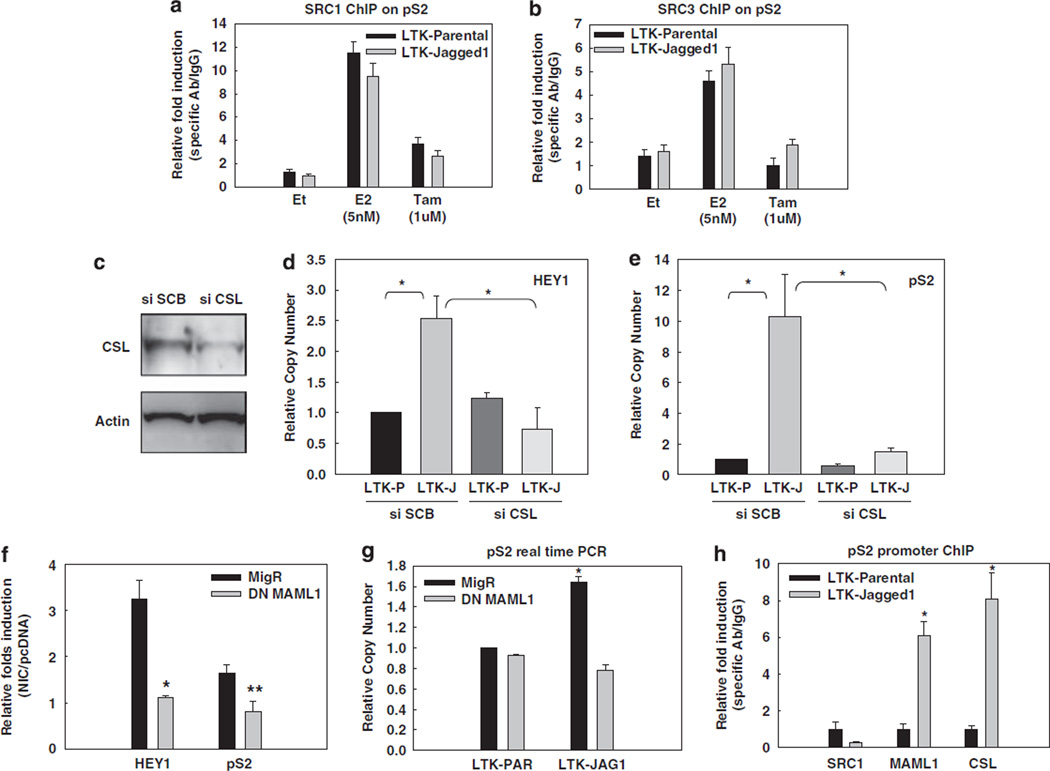
Figure 4.
SRC-1 or SRC-3 are not required, but CSL and MAML1 are indispensable for the effect of Notch-1 on the pS2 promoter. In all experiments, MCF-7 cells were grown in charcoal-stripped medium for a total of 3 days. SRC-1 (a) and SRC-3 (b) were detected by ChIP on the pS2 promoter. Charcoal-stripped MCF-7 cells were co-cultured with LTK–JAG1 or LTK–PAR fibroblasts for 3 h, and treated with 5 nm E2 (1 h) or 1 µm 4-hydroxytamoxifen (24 h); *P ⩽ 0.001. (c) MCF-7 cells were transfected with CSL siRNA or scrambled control. CSL knockdown was verified by western blotting. (d, e) HEY1 and pS2 real-time RT–PCR was performed with cells transfected with CSL siRNA and co-cultured with LTK fibroblasts. (f) MCF-7 cells were co-transfected with NIC or pcDNA control and DN-MAML1 or the empty vector MigR. HEY1 and pS2 real-time RT–PCR was performed 48 h after transfection. Data are expressed as relative fold induction by NIC over pcDNA (*P ⩽ 0.001, **P ⩽ 0.005). (g) MCF-7 cells were transfected with DN-MAML1 or the empty vector MigR under charcoal-stripped conditions and co-cultured with LTK cells for 12 h. pS2 mRNA level was measured by real-time RT–PCR; *P ⩽ 0.001. (h) ChIP–PCR of SRC1, MAML1, CSL on pS2 promoter with MCF-7 cells co-cultured with LTK–JAG1 or LTK–PAR fibroblasts; *P ⩽ 0.001. ChIP, chromatin immunoprecipitation; DN-MAML1, the dominant-negative form of MAML1; GSI, g-secretase inhibitor; LTK–JAG1, mouse LTK fibroblasts expressing Notched ligand Jagged-1; LTK–PAR, control vector-transduced parental fibroblast; NIC, active form of Notch-1 (Notch-1IC); RT–PCR, reverse transcription–PCR; siRNA, short interfering RNA; SRC, steroid receptor coactivator.