Abstract
The development of the fetal immune system during pregnancy is a well-orchestrated process with important consequences for fetal and neonatal health, but prenatal factors that affect immune activation are poorly understood. We hypothesized that chronic fetal inflammation may lead to alterations in the development of the fetal immune system. To test this hypothesis, we examined neonates with gastroschisis, a congenital abdominal wall defect that leads to exposure of the fetal intestines to amniotic fluid, with resultant intestinal inflammation. We determined that patients with gastroschisis show high systemic levels of inflammatory cytokines and chemokines such as eotaxin, and earlier activation of CD4+ and CD8+ effector and memory T cells in the cord blood compared to controls. In addition, increased numbers of T cells and eosinophils infiltrate the serosa and mucosa of the inflamed intestines. Using a mouse model of gastroschisis, we observed higher numbers of eosinophils and both type 2 and type 3 innate lymphoid cells (ILC2 and ILC3), specifically in the portion of organs exposed to the amniotic fluid. Given the role of IL-5 produced by ILC2 in regulating eosinophil development and survival, we determined that maternal or fetal administration of the anti IL-5 neutralizing antibody, or a depleting antibody against ILCs, can both effectively reduce intestinal eosinophilia. Thus, a congenital anomaly causing chronic inflammation can alter the composition of circulating and tissue-resident fetal immune cells. Given the high rate of prenatal and neonatal complications in these patients, such changes have clinical significance and might become targets for fetal therapy.
Introduction
Human fetal immune development is a critical component of pre- and post-natal health, yet the environmental factors governing normal immune ontogeny remain poorly understood. There is a growing understanding of prenatal factors that may impact immune development. For example, exposure to retinoic acid is important in the development of lymphoid tissue inducer cells (LTi) (1). Furthermore, increasing evidence indicates that the fetal adaptive immune system is more sophisticated than previously recognized. For example, the neonatal intestine contains memory T cells that may facilitate mother-to-child HIV transmission (2) and circulating neonatal T cells can contribute to ongoing inflammation by producing chemokines such as IL-8 (3). However, whether and how fetal immune development is altered in patients with birth defects that change this developmental milieu is not understood.
Gastroschisis is a congenital defect of the abdominal wall that results in exposure of the intestines to amniotic fluid during development. Although the etiology is not known, gastroschisis is characterized by inflammation of the exposed intestines, with resultant bowel damage (4). Since this defect develops early in gestation, it represents a natural model in which to examine the consequences of chronic inflammation on the development of systemic and tissue-resident fetal immune cells. Several groups have examined amniotic fluid from these pregnancies and found higher levels of IL-6 and IL-8, with concomitant increases in mononuclear cells and neutrophils in patients with gastroschisis compared to controls (5, 6). The current understanding of this disease is that exposure to the inflammatory cytokines in amniotic fluid, in addition to possible ischemic damage from a small abdominal wall defect, activates an innate immune response in the serosa of the exposed intestine. However, systemic immune changes in the fetus have not yet been examined.
Studying immune changes in gastroschisis has important clinical relevance. Pregnancies affected with fetal gastroschisis can have multiple complications such as low fetal weight, in utero fetal demise, or preterm delivery (7, 8). Although the intestines can be placed back into the abdomen after birth, neonates with gastroschisis often suffer from poor intestinal motility after this operation and spend at least several weeks in the neonatal intensive care unit until the bowel recovers enough to tolerate enteral feeds. It is possible that alterations in fetal immune development may play a role in these related problems, and if so, targeted treatments could be developed.
To explore the potential roles of prenatal inflammation and fetal immune activation in gastroschisis, we examined cytokine profiles and relevant cell types in cord blood and intestines of neonates with this disease. We also used a genetic mouse model of gastroschisis (9) to confirm these findings and to test fetal treatment strategies. Our results indicate significant changes in both innate and adaptive immune cell development in gastroschisis, which may impact downstream complications in these patients. Importantly, we demonstrate that some of these changes can be reversed with fetal therapy.
Materials and Methods
Subjects
Mothers carrying unaffected fetuses, those with gastroschisis, or those with a large omphalocele (an abdominal wall defect in which the intestines are still covered and do not demonstrate inflammation) were prospectively enrolled between November 2009 and December 2012. The Institutional Review Board at the University of California approved the study and written informed consent was obtained.
Human blood sample processing
Samples of cord blood were collected at the time of birth and maternal blood was collected within 24 hours of delivery. Plasma was frozen and stored for batch analysis of cytokines. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque PLUS density gradient centrifugation, frozen, and analyzed in batches during the study period.
Cytokine assay
Cytokine profiles in the maternal and cord blood plasma samples were assayed using the standard-sensitivity Millipex Map kit (Millipore) as previously reported (10). Samples were acquired and analyzed on a Labscan 100 analyzer (Luminex) using Bio-Plex manager 6.0 software (Bio-Rad).
Preparation of human intestinal samples
The UCSF pathology database was queried after IRB approval to identify patients with gastroschisis who underwent bowel resection between February 2004 and April 2012. Patients who underwent surgical resection secondary to intestinal obstruction or atresia (and not bowel necrosis) and had a lymphocytic infiltrate on hematoxylin and eosin (H&E) stain were identified for further analysis and immunostaining. Control specimens were obtained from patients undergoing autopsy for birth asphyxia or surgical resection specimens from patients without gastroschisis, taking unaffected portions of intestine. 5μm sections from formalin-fixed, paraffin-embedded tissues were stained using an antibody against human CD3 (F7.2.38; Dako) and counterstained with Hematoxylin to identify T cells. Eosinophils were identified using H&E staining (11). Cell counts were performed on random high-power fields, excluding areas of lymphoid follicles, by a pathologist (ANM) blinded to patient group. Fresh bowel specimens were collected from surgical procedures in three gastroschisis patients and four controls. The intra-epithelial (IEL) and lamina propria lymphocytes (LPL) were isolated from the mucosa and submucosa, respectively, as previously described (12). The mucosa was dissected from the muscular layer and washed with 1mM DL-Dithiothreitol (DTT) followed by 5mM EDTA to isolate IEL. The remaining tissue was mechanically dissociated, then digested with 0.1 mg/mL of collagenase to harvest LPL.
Mice
Aortic carboxypeptidase-like protein (ACLP)+/− mice (9) were obtained from Dr. Matthew Layne (Harvard Medical School). Mice were bred and maintained in a specific pathogen-free facility at UCSF. All mouse experiments were performed according to the UCSF Institutional Animal Care and Use Committee approved protocol. ACLP+/− mice were bred to each other and all pups were harvested at E18.5. Lymphocytes were isolated from spleens, livers, and intestines of ACLP−/− pups with gastroschisis and unaffected littermate controls. Liver was lysed using ACK lysing buffer. Intestine was mechanically dissociated and digested first with 10mM DTT, then with 1mg/mL dispase and 100U/mL DNase I solution.
Fetal blockade of IL-5
ACLP+/− pregnant females were injected intravenously with a neutralizing antibody against IL-5, (TRFK5; BD Pharmingen) or an isotype control (R3-34; BD Pharmingen) for five consecutive days, using a dose of 100 μγ on E13.5-E14.5 and 150 μγ on E15.5-E17.5. The amount of antibody was calculated to achieve a fetal dose of 10mg/kg (the same dose that is typically used in an adult mouse (13)) and an estimated maternal-fetal transmission of 2-3% (14). Fetal intestines and liver were analyzed on E18.5. In the case of fetal intervention, fetuses were injected with the same antibodies on E13.5 using a fetal intrahepatic injection as previously described (15-17). Briefly, mothers were anesthetized and the uterus was exposed by performing a laparotomy. The fetuses were injected individually, through the translucent intact uterus, with the neutralizing antibody; controls were injected with the isotype control antibody or PBS in a volume of 5 μl, using pulled glass micropipettes. The laparotomy was closed in layers and the fetuses were analyzed on E18.5.
Antibody-mediated depletion of fetal ILCs
ACLP+/− pregnant females were bred to ACLP+/− males and the fetuses were injected on E13.5 with an anti-Thy1.2 antibody (30H12; BioXCell) or isotype control (LTF-2; BioXCell) directly into the fetal liver as described above. The pups were harvested on E18.5 and intestines were processed and stained for flow cytometry.
Flow cytometry analysis
Human cells
We stained human PBMCs with monoclonal antibodies to CD3 (UCHT1; BD Biosciences), CD4 (SK3; BD Biosciences), CD8 (SK1; BD Biosciences), CD25 (M-A251; BD Biosciences), CD45RA (ALB11; Beckman Coulter), CCR7 (MAB197; R&D Systems), Helios (22F6; BioLegend), Foxp3 (236A/E7; eBioscience), IL4 (MP4-25D2; BD Biosciences), IL-17 (eBio64DEC17; eBioscience), IFN-γ (B27; BD Biosciences) and a Fixable Viability Dye eFluor780 (eBioscience). The cytokine-producing capacity of T cell subsets was assessed after PBMCs were stimulated (at a density of 5 × 105 cells per 100 μL) for 5 h with 50 ng/ml PMA (phorbol 12-myristate 13-acetate; Sigma-Aldrich) and 2 mg/ml of ionomycin (Sigma-Aldrich) in the presence of 10 mg/ml of brefeldin A (Sigma-Aldrich). Cells were fixed and made permeable with the Foxp3/Transcription Factor Staining Buffer Set according to the manufacturer's instructions (eBioscience). For flow cytometric analysis of human intestine, IEL and LPL were stained with monoclonal antibodies to CD3 (UCHT1; BD Biosciences), CD4 (SK3; BD Biosciences), CD8 (SK1; BD Biosciences), IL-7Rα (HIL-7R-M21; BD Pharmingen), and c-Kit (104D2; BD Biosciences).
Mouse cells
We stained mouse leukocytes with monoclonal antibodies to CD3 (17A2; BioLegend), TCRβ (H57-597; eBioscience), TCRγ/δ (GL3; BioLegend), CD5 (53-7.3; eBioscience), CD19 (6D5; BioLegend), CD11b (M1/70; BD Pharmingen), CD11c (N418; eBioscience), NK1.1 (PK136; eBiosciences), CD45.2 (104; eBioscience), Thy1.2 (53-2.1; BioLegend), CD8a (53-6.7, BD Pharmingen), CD4 (RM4-5; BD Pharmingen), RORγt (B2D; eBioscience), Gata-3 (TWAJ; eBioscience), c-kit (2B8; eBioscience), IL-7Rα (A7R34; eBioscience), Siglec-F (E50-2440; BD Pharmingen) and Fixable Viability Dye eFluor780 (eBioscience). All samples were acquired with an LSR II (Becton Dickinson) flow cytometer and analyzed with FlowJo software (Treestar Inc.).
Reagents
Reagents included Ficoll-Paque PLUS (GE Healthcare), ACK lysing buffer (Lonza), DL-Dithiothreitol (AllStar Scientific), Ethylenediaminetetraacetic acid (EDTA, Corning Cellgro), Collagenase (from Clostridium histolyticum; Sigma), Dispase (Gibco), and DNase I (bovine pancreas; Roche).
Statistical Analysis
Statistical analysis and graphs were generated using Prism5 software (GraphPad Software) or Stata version 12 (Stata Corp LP, College Station, TX). Data were analyzed using the Mann-Whitney U test (for nonparametric data), unpaired t test (for normally distributed data), or Chi square test (to assess the differences in proportion between groups). For multiple categories, one-way ANOVA with Tukey's post-test was applied. For cytokine analysis, a supervised Principal Component Analysis (sPCA) was performed, which used the subset of cytokines that were most correlated with the outcome (Spearman correlation coefficient >0.25). The Kendall tau-c statistic was also calculated to test the magnitude and direction of increases or decreases in cytokine levels across disease severity groups. A multiple regression analysis was performed to account for the presence of spontaneous preterm labor and maternal smoking (data on smoking was not recorded in the medical record for 2/30 controls and 4/26 patients with GS; these patients were analyzed as non-smokers). A p-value <0.05 was considered statistically significant.
Results
Levels of inflammatory mediators are increased in the cord blood of patients with gastroschisis
We prospectively enrolled 27 patients with gastroschisis and 23 healthy term controls for collection of maternal and cord blood at the time of delivery (Table I). There were no significant differences in nulliparity, mode of delivery, and fetal gender between mothers of healthy controls and those of fetuses with gastroschisis. Mothers of fetuses with gastroschisis were more likely to be younger, experience preterm labor, deliver earlier, and have infants with lower birth weights than were mothers with unaffected pregnancies, as previously reported (7). Other clinical characteristics of patients with GS are shown in Supplementary Table 1.
Table I.
Demographic characteristics of healthy controls and patients with gastroschisis.
| Control (n=30) | Gastroschisis (n=27) | P value | |
|---|---|---|---|
| Maternal age (yrs) | 32 (18-43) | 23 (15-34) | <0.005 |
| Maternal smoking | 0 (0%) | 6 (22.2%) | 0.004 |
| Nulliparous | 9 (30.0%) | 14 (51.9%) | 0.09 |
| Mode of delivery | |||
| Vaginal | 21 (70.0%) | 23 (85.2%) | 0.17 |
| C-section | 9 (30.0%) | 4 (14.8%) | 0.17 |
| Presence of labor | 26 (86.7%) | 27 (100.0%) | 0.05 |
| Gestational age (weeks) | 39.7 (37.1-41.6) | 36.3 (34.7-37.4) | <0.005 |
| Male fetus | 15 (50.0%) | 18 (66.7%) | 0.20 |
| Birth weight (g) | 3535 (2680-4440) | 2495 (1730-3130) | <0.005 |
| Preterm labor | 0 (0%) | 9 (33.3%) | 0.001 |
Data presented as average (range) or n (%). P-value was calculated using student's t-test or chi squared test.
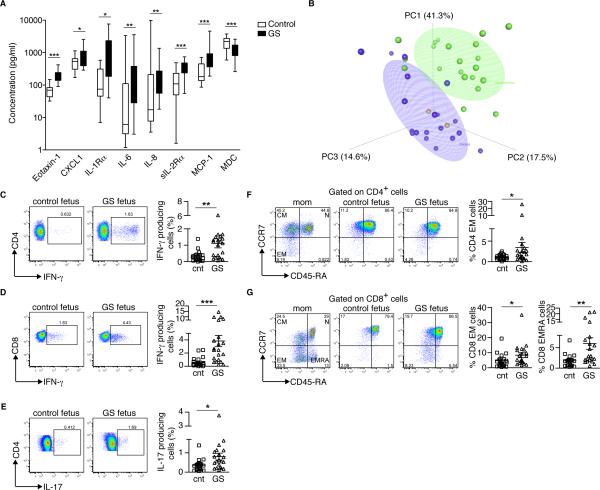
The amniotic fluid in patients with gastroschisis has increased levels of inflammatory cytokines (5, 6). To determine whether there is evidence of fetal systemic inflammation in this condition, we analyzed serum cytokines in cord blood. We found that fetuses with gastroschisis had significantly elevated levels of eotaxin-1 (CCL11), CXCL1, IL-1 receptor α (IL-1Rα), IL-6, IL-8, soluble IL-2 receptor α (sIL-2Rα), and Monocyte Chemoattractant Protein-1 (MCP-1), and decreased levels of Macrophage-Derived Chemokine (MDC) in the cord blood compared to controls (Fig. 1A; results for all cytokines tested and all relevant comparisons are shown in Supplementary Table II). We also performed a supervised Principle Components Analysis (sPCA) using the plasma biomarkers that were correlated with gastroschisis (those with Spearman correlation coefficient >0.25; namely, eotaxin, CXCL1, IL-1Rα, IL-6, IL-8, sIL-2Rα, MCP-1, MDC, IL-12 (p40), and TGFα). The first principal component predominantly comprised eotaxin, IL-1Rα, IL-6, IL-8, and MCP-1, and differed the most between patients and controls (Fig. 1B). Of note, we also analyzed two patients who had a large omphalocele (an abdominal wall defect in which the intestine is covered with amnion and does not exhibit inflammation) and their cytokine profiles were within the control distribution (Fig. 1B). Thus, there is evidence of systemic inflammation in fetuses with gastroschisis.
FIGURE 1.
Elevated inflammatory markers and T cell activation in the cord blood of patients with gastroschisis. (A) Comparison of cytokine levels in unaffected controls (white, n=19) and patients with gastroschisis (black, n=21). The horizontal line represents the median for each group. *p=0.05; **p<0.01; ***p<0.001 by Mann-Whitney rank sum test. MCP-1, Monocyte Chemoattractant Protein-1; MDC, Macrophage-Derived Chemokine. (B) Supervised Principal Component Analysis (sPCA) of the cord blood biomarkers most significantly correlated with gastroschisis in healthy controls (n=19; blue), patients with gastroschisis (n=21; green) and patients with giant omphalocele (n=2; orange). Ellipsoids represent a 50% concentration of observations for controls and patients with gastroschisis. Eotaxin-1, IL-1Rα, IL-6, IL-8 and MCP-1 had the highest factor loadings on PC1. (C-E) Cord blood PBMCs were stimulated with PMA and Ionomycin and stained with antibodies to CD3, CD4, CD8, IL-17 and IFN-γ to determine the percentage of T cells producing each cytokine. (C) IFN-γ production by CD4+ T cells. (D) IFN-γ production by CD8+ T cells. (E) IL-17 production by CD4+ T cells. Each symbol represents a single patient; small horizontal bars indicate the mean. (F and G) On the left side, representative flow cytometric analysis of purified maternal and cord blood PBMCs from healthy controls and patients with gastroschisis stained with antibodies to CD3, CD4, CD8, CD45RA and CCR7 to detect naïve (N: CCR7+CD45RA+), central memory (CM: CCR7+CD45RA−) and effector memory (EM: CCR7−CD45RA−; EMRA: CCR7−CD45RA+) cells. On the right side, compiled analysis for cord blood (F) CD4+ EM T cells, (G) CD8+ EM T cells and CD8+ EMRA T cells. Each symbol represents a single patient; small horizontal bars indicate the mean. Control n=20, gastroschisis n=21. *p<0.05; **p<0.01; ***p<0.001 by Mann-Whitney test.
Pregnancies with GS have a higher incidence of spontaneous preterm labor (sPTL) and maternal smoking. To determine whether the observed changes in cytokines were secondary to PTL or maternal smoking, we performed a multiple regression analysis and adjusted for these variables. All of the observed cord blood cytokine changes remained statistically significant even when controlling for PTL and maternal smoking, suggesting these changes are secondary to the underlying disease.
Early development of effector T cells in patients with gastroschisis
We next asked whether fetal immune development was altered in the context of systemic inflammation. We focused first on T cells, given their role in promoting intestinal inflammation in other settings (18-20). We found that patients with gastroschisis have significantly increased percentages of CD4+ and CD8+ T cells that produce IFN-γ compared to controls (Fig. 1C, 1D); the increase was most pronounced in CD8+ T cells. The numbers of Th17 cells in patients with gastroschisis also increased (Fig. 1E), although their concentration in the blood is usually relatively low (21). These results are consistent with the systemic increase in IL-6, which can control the differentiation of T cells to Th17 cells (21). However, we did not observe a difference in the percentages of IL-4 producing (Th2) fetal T cells (Supplementary Fig. 1A). We also examined the percentages of naïve (N, CD45RA+CCR7+), central memory (CM, CD45RA−CCR7+), effector memory (EM, CD45RA−CCR7−), and terminally differentiated effector memory (EMRA, CCR7−CD45RA+) cells (22) and found increased percentages of CD4+ EM and CD8+ EMRA T cells in patients with gastroschisis; once again, the increases were most pronounced (and likely biologically relevant) among CD8+ T cells. (Fig. 1F, 1G). When we adjusted for the presence of sPTL, the changes in CD4 and CD8 cells producing IFNγ and CD8 EM and CD8 EMRA cells remained significant. Finally, given previous reports of the propensity of fetal T cells to differentiate into regulatory T cells (23), we compared percentages of Foxp3+Helios+ Tregs in patients and controls and found no differences (Supplementary Fig. 1B, 1C). Thus, chronic inflammation in gastroschisis leads to the production of effector T cells without a compensatory regulatory response.
Immune alteration in gastroschisis is manifested predominantly on the fetal, not maternal side
To determine whether there were maternal immune changes in pregnancies affected with gastroschisis, we examined plasma cytokine and blood T cell phenotypes in matched maternal samples collected at the time of delivery. We detected a generalized decrease in cytokine, chemokine, and growth factor levels in women carrying fetuses with gastroschisis compared to controls, with significant decreases in levels of fractalkine, GM-CSF, and IL-12 (p70) (Supplementary Table III). In examining circulating T cell profiles, we detected a significant increase in Th17 cells (Supplementary Fig. 1D), but no differences in the production of IFN-γ βψ XΔ4+ ○ρ XΔ8+ T χελλσ (Supplementary Fig. 1E, 1F) in mothers of gastroschisis patients. However, the increase in Th17 cells was not significant after adjusting for sPTL. We also observed that maternal CD4+ EM T cells increased slightly, but CD8+ EM and EMRA T cells did not change in pregnancies with gastroschisis (Supplementary Fig. 1G). Thus, immune activation in this disease occurs predominantly on the fetal side.
Infiltration of the intestine by T cells and eosinophils in patients with gastroschisis
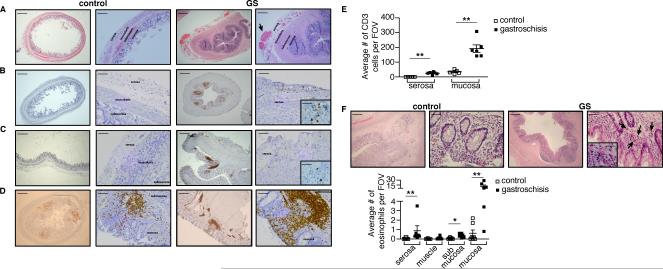
The systemic increases in activated T cells led us to evaluate whether similar changes could be found locally in the inflamed intestines. Neonates with gastroschisis have an inflammatory peel surrounding the intestine, characterized by subserosal edema, fibrosis, macrophage infiltration, capillary proliferation, and epithelial cell deposition, which develops after 30 weeks of gestation (24), but T cell infiltration has not been evaluated. We examined surgical specimens of non-necrotic intestine in patients with gastroschisis and age-matched controls using H&E, and stained for T cells using an antibody against CD3. We confirmed that the intestine of patients with gastroschisis is characterized by a marked thickening of all layers and serosal vascular proliferation, suggesting chronic ischemic injury (Fig. 2A). T cells were present in the intestinal serosa (Fig. 2B, 2C) and lymphoid follicles in patients with gastroschisis (Fig. 2D), with infiltration beyond the boundaries of these follicles into the submucosa and mucosa, which was not seen in healthy controls. Overall, patients with gastroschisis showed significantly higher numbers of T cells in the intestinal mucosa as well as the serosa (Fig. 2E). Thus, histologic changes in the intestine extend well beyond the serosal surface that is exposed to amniotic fluid and are more consistent with systemic immune alterations.
FIGURE 2.
Infiltration of T cells and eosinophils into the intestine of patients with gastroschisis. Histological analysis of bowel specimens from healthy controls (left panels) and patients with gastroschisis (right panels). (A) Hematoxylin and eosin staining of appendix sections shows the presence of numerous vessels in the serosa in patients with gastroschisis. Lower magnification bars, 500 μm. Higher magnification bars, 100 μm. (B and C) Immunohistochemical staining of (B) appendix and (C) colon sections shows infiltration of CD3+ T cells (brown) in the serosa of specimens from patients with gastroschisis. Lower magnification bars, 500 μm. Higher magnification bars, 100 μm and inserts, 20 μm. (D) Enlarged lymphoid follicles and infiltration of CD3+ T cells (brown) in the mucosa of colon in patients with gastroschisis. Lower magnification bars, 500 μm. Higher magnification bars, 100 μm. (E) Numbers of CD3+ T cells infiltrating the intestine per field of view (FOV), excluding lymphoid follicles. Control n=5, gastroschisis n=6. (F) Hematoxylin and eosin staining shows the presence of eosinophils (indicated by the arrows) infiltrating the mucosa in colon of patients with gastroschisis. Control n=7, gastroschisis n=6. Lower magnification bars, 200 μm. Higher magnification bars, 50 μm and insert, 20 μm. The graph shows the numbers of eosinophils infiltrating the intestine. **p<0.01 by Mann-Whitney test.
Since eotaxin-1 was increased in cord blood plasma of patients with gastroschisis, we also examined eosinophils in bowel samples of these neonates. Activated eosinophils have been implicated in intestinal inflammation and dysfunction in other clinical settings (25) but have not been examined previously in this disease. We found that eosinophils were dramatically increased in the intestinal mucosa of patients with gastroschisis compared to age-matched controls (Fig. 2F).
Altered immune development in fetal mice with gastroschisis
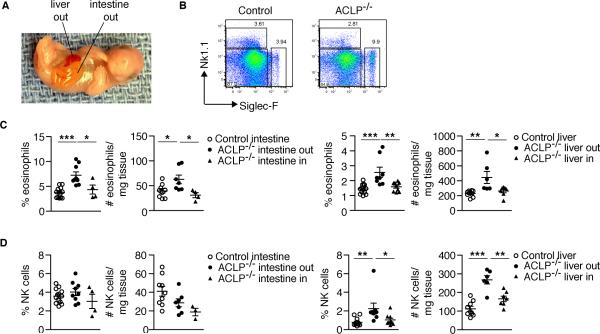
Given the interesting changes in fetal immune development in patients with an abdominal wall defect, we next turned to an appropriate mouse model to validate these findings and test prenatal treatment strategies. Mice lacking the aortic carboxypeptidase-like protein (ACLP) (9) have a spontaneous abdominal wall defect with exteriorized bowel and liver that results in decreased numbers of enteric neurons and interstitial cells of Cajal (26), similar to human gastroschisis (Fig. 3A). To characterize the fetal immune response in this mouse model, we examined cells of both the adaptive and innate immune systems in affected fetuses (ACLP−/−) and their unaffected littermates (ACLP+/− and ACLP+/+) at E18.5. We detected elevated eosinophils (both the percentages and absolute numbers) in the fetal mouse intestine and liver (Fig. 3B, 3C). There were also increased NK cells in the exteriorized liver (Fig. 3D). Importantly, the changes in the affected mice were observed predominantly in herniated organs exposed to amniotic fluid and were not seen in non-herniated organs (Fig. 3C, 3D) or in the spleen (data not shown). T cells were unchanged (data not shown), likely secondary to their delayed maturation in mice compared to humans. Despite differences in the timing of fetal immune development between mouse and human, the finding of altered immune maturation in the mouse indicates that the ACLP−/− mouse is a suitable model for mechanistic studies on bowel damage in gastroschisis.
FIGURE 3.
Increased eosinophils and NK cells in the exteriorized intestines and livers of ACLP−/− mice. (A) E18.5 ACLP−/− mouse exhibiting a defect in the anterior abdominal wall with exteriorized liver and intestine. (B) Representative flow cytometric analysis of NK cells (Nk1.1+) and eosinophils (Siglec-F+) from the intestine of E18.5 littermate controls (control) and ACLP−/− mice after first gating on CD45.2+ leukocytes. (C and D) Histograms showing the percentages and absolute cell number/mg tissue of (C) eosinophils and (D) NK cells in the exposed (out) or non-exposed (in) intestine and fetal liver. Each symbol represents a single fetus; small horizontal bars indicate the mean. Control n=13, ACLP−/− n=9. *p<0.05; **p<0.01; ***p<0.001 by one-way ANOVA with Tukey's post-tests.
Innate lymphoid cells accumulate in the intestines of patients and mice with gastroschisis
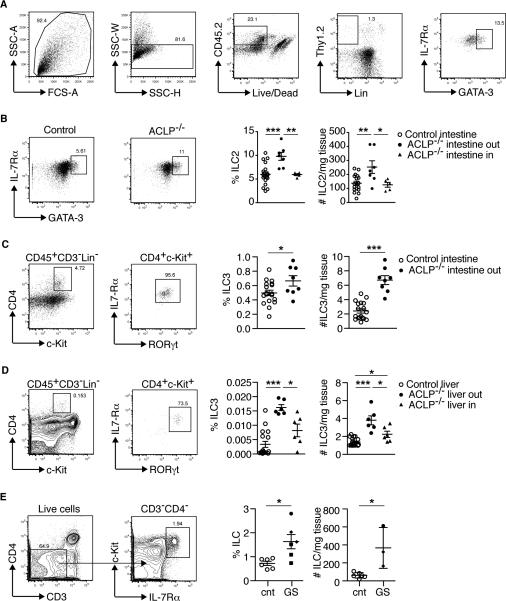
Innate lymphoid cells (ILCs) are crucial determinants of fetal immune development (27, 28), especially in the fetal intestine (29). In particular, eosinophil development and survival is mediated by interleukin (IL)-5, the serum levels of which are maintained by tissue-resident ILC2 (30). Since eosinophils were increased in mice with gastroschisis, we examined ILC2 in the intestines of ACLP−/− mice and found that both percentages and absolute numbers of ILC2 are increased in the exteriorized intestine compared to littermate controls (Fig. 4A, 4B). Another subset of innate lymphoid cells, ILC3, has been shown to contribute to the inflammatory state of the intestine during pathological conditions (31). Therefore, we analyzed ILC3 and found that both the percentages and absolute numbers of ILC3 (defined as CD45+Lin−CD3−CD4+ckit+IL-7Rα+RORγt+) (32) were also increased in the exposed intestine (Fig. 4C) and livers (Fig. 4D) of ACLP−/− mice compared to littermate controls. We also examined ILCs in intestines of neonates with gastroschisis. Our numbers were limited by the rarity of intestinal resection—and therefore of fresh tissues—in these patients, so we performed a general stain for ILC that includes the different subsets (CD3−CD4−c-kit+IL-7Rα+) (32) after isolating intraepithelial and lamina propria lymphocytes. We detected increased numbers of ILC in the intestines of patients with gastroschisis compared to controls (Fig. 4E).
FIGURE 4.
Increased innate lymphoid cells (ILCs) in the exteriorized organs of ACLP−/− mice and patients with gastroschisis. (A) Sequential gating strategy to identify ILC2 (defined as CD45+Lin−CD3−Thy1.2+IL-7Rα+GATA-3+) in the intestine of a representative ACLP−/− mouse. (B) Representative flow cytometric plot of ILC2 and graphs showing the percentages and absolute cell number/mg tissue in the exposed (out) or non-exposed (in) intestine in littermate controls and ACLP−/− mice. Each symbol represents a single fetus; small horizontal bars indicate the mean. Control n=18, ACLP−/− n=12. *p<0.05; **p<0.01; ***p<0.001 by one-way ANOVA. (C and D) Gating strategy to identify ILC3 (defined as CD45+Lin−CD3−CD4+IL-7Rα+c-Kit+RORγt+) from E18.5 ACLP−/− mouse (example shown) and graphs showing the percentages (on total CD45+ cells) and absolute cell number/mg tissue in the exposed (out) or non-exposed (in) (C) intestine and (D) fetal liver in littermate controls and ACLP−/− mice. In this set of experiments, the entire intestine was exteriorized in the affected mice. Control n=6, ACLP−/− n=7. *p<0.05; **p<0.01; ***p<0.001 by one-way ANOVA. (E) Flow cytometric analysis of ILC (defined as CD3−CD4−IL-7Rα+c-Kit+) and compiled analysis of ILC of lamina propria and intraepithelial lymphocytes isolated from intestine of healthy controls (cnt, n=6 samples from 4 patients) and patients with gastroschisis (GS, n=6 samples from 3 patients). Among gastroschisis samples, each symbol (square, diamond and circle) represents a different patient; small horizontal bars indicate the mean. *p<0.05 by Mann-Whitney test.
Fetal therapy with a neutralizing antibody against IL5 can reverse the inflammatory infiltrate in ACLP −/− mice
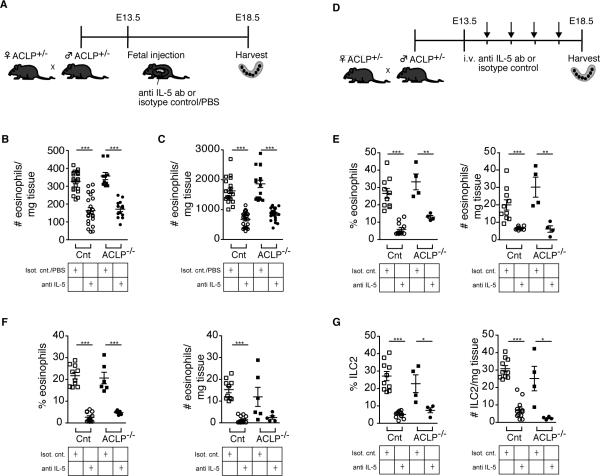
We next sought to define a treatment that could address some of the immunologic changes seen in the mouse model of gastroschisis. Since eosinophils and ILC2 were increased in this model, we targeted IL-5 signaling and tested both direct fetal injection and maternal administration of a blocking antibody. We first tested direct fetal administration by injecting fetal mice with TRFK5, a neutralizing antibody against IL-5, or either isotype control or PBS on E13.5-E14.5, using our established fetal intrahepatic injection method (15-17). We then harvested the pups on E18.5 and analyzed the immune cells in exposed and non-exposed organs (Fig. 5A). These experiments showed that all fetuses injected with the isotype control or PBS had high levels of eosinophils, indicating that sterile inflammation from the surgery results in a fetal immune response. However, this rise in eosinophils was mitigated effectively by blocking IL-5 in both the intestine and the liver (Fig. 5B, 5C).
FIGURE 5.
Decreased eosinophil infiltration and ILC2 in ACLP−/− mice after treatment with anti IL-5 antibody. (A) Schematic layout of the experiment. Fetal intestine and liver are harvested 5 days after fetal liver injection of anti IL-5 or isotype control/PBS. (B and C) Histograms showing the absolute cell number/mg tissue of eosinophils in the intestine (B) and fetal liver (C). Each symbol represents a single fetus; small horizontal bars indicate the mean. Control n=43, ACLP−/− n=23. ***p<0.001 by Student's t test. (D) Schematic layout of the experiment. Maternal intravenous injection of anti IL-5 or isotype control daily between E13.5-E18.5 and harvest of fetal intestine and liver on E18.5. (E and F) Histograms showing the percentages and absolute cell number/mg tissue of eosinophils in the intestine (E) and fetal liver (F). (G) Histograms showing the percentages and absolute cell number/mg tissue of ILC2 in the intestine of littermate control and ACLP−/− mice. Each symbol represents a single fetus; small horizontal bars indicate the mean. Control n=14, ACLP−/− n=8. *p<0.05; **p<0.01; ***p<0.001 by Student's t test.
We next examined maternal administration of TRFK5, since this antibody can cross the placenta. Maternal intravenous injections of TRFK5 (or isotype control) were given daily between E13.5-E17.5, and fetal intestine and liver were harvested on E18.5 (Fig. 5D). This treatment led to a significant decrease in eosinophils compared to isotype control in both the intestine (Fig. 5E) and liver (Fig. 5F). The treatment was effective in reducing eosinophil counts in both gastroschisis and control mice; ILC2 were reduced as well (Fig. 5G).
Depletion of ILCs can ameliorate intestinal eosinophilia in ACLP−/− mice
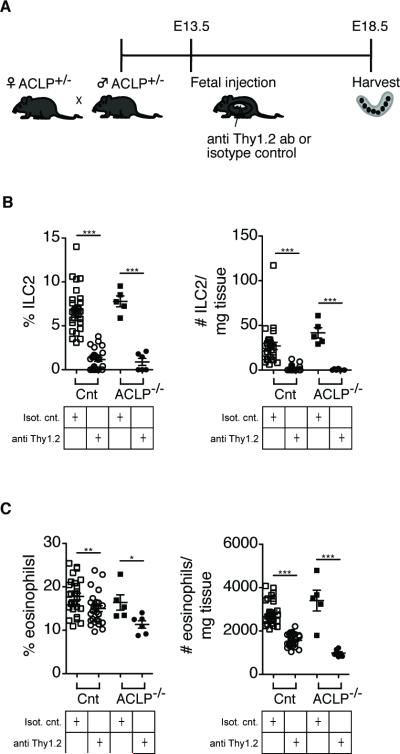
ILC2 have been implicated in the onset of eosinophilia by producing IL5 (33). To determine whether there is a mechanistic link between ILC2 and eosinophilia in our model, we depleted fetal ILCs by injecting fetuses with a depleting antibody against Thy1.2 on E13.5 and harvested the pups on E18.5 (Fig. 6A). We detected a significant decrease in the percentages and absolute numbers of ILC2 and eosinophils in the intestines of treated pups compared to those treated with an isotype control (Fig. 6B, 6C). Thus, ILC2 may be involved in recruiting eosinophils to the intestine in gastroschisis. Collectively, our results indicate that targeted prenatal therapies may limit ongoing fetal inflammation and aberrant immune development during prenatal inflammation.
FIGURE 6.
Decreased eosinophil infiltration in ACLP−/− mice after fetal treatment with anti Thy1.2 antibody to deplete innate lymphoid cells (ILC). (A) Schematic layout of the experiment. Fetal mice are injected with anti Thy1.2 or isotype control antibody and harvested on E18.5. (B) Histograms showing the depletion of ILC2 (% and absolute cell number/mg tissue) in the intestine. (C) Histograms showing the reduction of eosinophils (% and absolute cell number/mg tissue) in the intestine. Each symbol represents a single fetus; small horizontal bars indicate the mean. Control n=56, ACLP−/− n=11. *p<0.05; **p<0.01; ***p<0.001 by Student's t test.
Discussion
In this study, we examined fetal immune development in the context of ongoing systemic inflammation, using a congenital abdominal wall defect as a model system. We demonstrate that patients with gastroschisis have increased systemic levels of inflammatory cytokines and specific changes in the development of effector T cell subsets. We also characterized changes in mucosal immunity in this disease, including local infiltration of T cells, eosinophils, and ILCs in the affected intestine. These changes were mirrored in the mouse model of gastroschisis and could be ameliorated by administration of an antibody against IL-5 or by depleting ILC2. Systemic changes in prenatal immune development as a result of chronic prenatal inflammation are a novel finding; our work suggests that these changes have consequences for the health of the fetus and neonate but could be reversed with in utero therapy.
Our findings add to the growing literature on human fetal immune development and indicate that the presence of external stressors prenatally can significantly affect the fetal immune system. It has been suggested that the initial insult in gastroschisis is ischemic, secondary to a narrow abdominal wall defect (34), and the cytokine profile we obtained is consistent with the changes reported in the setting of intestinal ischemia-reperfusion injury (35). We speculate that the ischemic insult initiates an inflammatory environment, leading to recruitment and activation of T cells and eosinophils and ultimately resulting in bowel damage. T cells have been found to infiltrate ischemic tissues and produce IL-17, which may mediate tissue damage (36), and T cell depletion can ameliorate intestinal damage in an intestinal ischemia-reperfusion injury model (37). ILC3 and γδ T cells may also contribute to the production of IL17, although their low numbers in circulation limited our ability to directly examine these cell types (38-40). Our data indicate that IL-5 production by ILC2 is involved in the recruitment of eosinophils in this context, but that prenatal blockade of IL-5 may dampen the fetal immune response.
Epithelial cells contribute to the cytokine milieu that stimulates ILC2 (41-44): thus, it is possible that the intestinal inflammation triggered by gastroschisis induces the expansion of ILC2s. Our data in the mouse model suggests that ILC2 can, in turn, contribute to increased intestinal eosinophilia. However, the role of ILCs in intestinal development is complex and there have also been reports that ILC2 promote tissue repair and homeostasis during inflammation (42, 45). It is possible that in patients with gastroschisis, ILC may also serve to quell inflammation, particularly since one of the hallmarks of this disease is spontaneous resolution of inflammation over several weeks/months after birth. Finally, since ILCs regulate T cell responses to commensal flora (46), alterations in their development may affect early microbial colonization. Of note, patients with gastroschisis are particularly susceptible to necrotizing enterocolitis, which has been associated changes in the microbiome (47) as well as dysregulation of intestinal T cells (12).
This report also adds to our understanding of the pathophysiology of intestinal damage in utero. Increases in tissue-resident eosinophils and T cells have been implicated in intestinal inflammation in other conditions and there may be similarities in the pathways leading to intestinal damage. For example, activated eosinophils and their granule proteins have been implicated in the pathogenesis of intestinal inflammation in inflammatory bowel disease (IBD), with increased levels correlating with worsening disease severity (48, 49). In addition to serving as a chemotactic factor for eosinophils, eotaxin may be involved in recruiting T cells that express the eotaxin receptor CCR3 to the intestine (50) and may adversely affect bowel motility (51). Locally, T cell infiltration and inflammatory cytokine production in the intestines may contribute to bowel dysmotility by multiple mechanisms, including damaging the interstitial cells of Cajal (52), which have been shown to be developmentally delayed in gastroschisis (53).
In addition to implications for the pathophysiology of bowel damage, the immune changes we observed may have implications for the health of the pregnancy. Patients with gastroschisis are susceptible to pregnancy complications such as preterm labor and intrauterine growth restriction and such complications may contribute to the cytokine and T cell profiles we observed in cord blood. Interestingly, all of the cytokine changes and some of the T cell changes remained significantly different when controlling for the presence of sPTL. It is possible that the underlying inflammation in gastroschisis (which is likely sterile, given the low rate of clinical chorioamnionitis observed) results in precocious activation of the fetal immune system and contribute to the onset of PTL (54). It will be interesting to determine the antigen specificity of activated fetal T cells to understand their functional significance. Understanding the consequences of specific fetal immune responses secondary to sterile inflammation (seen with this condition) or during prenatal infections could lead to valuable insights into reproductive immunology.
Although fetuses with gastroschisis are often diagnosed prenatally, there is currently no fetal therapy to decrease intestinal damage. Serial exchange of amniotic fluid has been proposed as a prenatal therapy to relieve bowel inflammation, without robust results (55), likely because there is ongoing fetal production of inflammatory mediators that act at the intestinal level to promote tissue damage. However, systemic treatments targeted at reducing particular cell types in utero may be a more effective option, as demonstrated by our mouse experiments. In this regard, further examinations into the role of IL-5 signaling in the prenatal period may yield clinically relevant therapeutic approaches for this disease.
Supplementary Material
Acknowledgements
We thank L. Coniglio, G. Bautista, and S. Trivedi for assistance with sample collection and processing, and our patients for their gracious participation in this study. We thank M. Layne (Department of Biochemistry, Boston University School of Medicine, Boston, MA, USA) for providing the ACLP+/− mice, P. Ursell (Department of Pathology, University of California San Francisco, San Francisco, CA, USA) for providing pathology specimens, and R. Locksley, J. Bando, M. McCune, C. Iqbal, and S. Fisher for helpful discussions.
Footnotes
This work was supported by grants from the March of Dimes (TCM), NIH/NIAID (K08AI085042 to TCM), and an American College of Surgeons Resident Research Scholarship (CJ).
Disclosures
The authors declare no competing financial interests.
REFERENCES
- 1.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labão-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O'Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunders MJ, van der Loos CM, Klarenbeek PL, van Hamme JL, Boer K, Wilde JCH, de Vries N, van Lier RAW, Kootstra N, Pals ST, Kuijpers TW. Memory CD4+CCR5+ T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood. 2012;120:4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons D, Fleming P, Virasami A, Michel M-L, Sebire NJ, Costeloe K, Carr R, Klein N, Hayday A. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med. 2014;20:1206–1210. doi: 10.1038/nm.3670. [DOI] [PubMed] [Google Scholar]
- 4.Christison-Lagay ER, Kelleher CM, Langer JC. Neonatal abdominal wall defects. Semin Fetal Neonatal Med. 2011;16:164–172. doi: 10.1016/j.siny.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Guibourdenche J, Berrebi D, Vuillard E, de Lagausie P, Aigrain Y, Oury J-F, Luton D. Biochemical investigations of bowel inflammation in gastroschisis. Pediatr Res. 2006;60:565–568. doi: 10.1203/01.pdr.0000242344.22638.94. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JJ, Klein N, Chitty LS, Kocjan G, Walshe D, Goulding M, Geary MP, Pierro A, Rodeck CH. Intra-amniotic inflammation in human gastroschisis: possible aetiology of postnatal bowel dysfunction. BJOG. 1998;105:1200–1204. doi: 10.1111/j.1471-0528.1998.tb09975.x. [DOI] [PubMed] [Google Scholar]
- 7.Barseghyan K, Aghajanian P, Miller DA. The prevalence of preterm births in pregnancies complicated with fetal gastroschisis. Arch Gynecol Obstet. 2012;286:889–892. doi: 10.1007/s00404-012-2394-3. [DOI] [PubMed] [Google Scholar]
- 8.Overcash RT, DeUgarte DA, Stephenson ML, Gutkin RM, Norton ME, Parmar S, Porto M, Poulain FR, Schrimmer DB. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551–557. doi: 10.1097/AOG.0000000000000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Layne MD, Yet SF, Maemura K, Hsieh CM, Bernfield M, Perrella MA, Lee ME. Impaired abdominal wall development and deficient wound healing in mice lacking aortic carboxypeptidase-like protein. Mol Cell Biol. 2001;21:5256–5261. doi: 10.1128/MCB.21.15.5256-5261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleck S, Bautista G, Keating SM, Lee T-H, Keller RL, Moon-Grady AJ, Gonzales K, Norris PJ, Busch MP, Kim CJ, Romero R, Lee H, Miniati D, MacKenzie TC. Fetal production of growth factors and inflammatory mediators predicts pulmonary hypertension in congenital diaphragmatic hernia. Pediatr Res. 2013;74:290–298. doi: 10.1038/pr.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown IS, Smith J, Rosty C. Gastrointestinal pathology in celiac disease: a case series of 150 consecutive newly diagnosed patients. Am J Clin Pathol. 2012;138:42–49. doi: 10.1309/AJCPE89ZPVJTSPWL. [DOI] [PubMed] [Google Scholar]
- 12.Weitkamp J-H, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, Oswald-Richter K, Rosen MJ, Engelhardt BG, Moore DJ, Polk DB. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013;62:73–82. doi: 10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Iijima K, Kita H. Marked airway eosinophilia prevents development of airway hyper-responsiveness during an allergic response in IL-5 transgenic mice. J Immunol. 2003;170:5756–5763. doi: 10.4049/jimmunol.170.11.5756. [DOI] [PubMed] [Google Scholar]
- 14.Medesan C, Cianga P, Mummert M, Stanescu D, Ghetie V, Ward ES. Comparative studies of rat IgG to further delineate the Fc : FcRn interaction site. Eur. J. Immunol. 1998;28:2092–2100. doi: 10.1002/(SICI)1521-4141(199807)28:07<2092::AID-IMMU2092>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Amar N, Tom L, Marta W, Tippi C M. A mouse model of in utero transplantation. J Vis Exp. 2011 doi: 10.3791/2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijagal A, Derderian C, Le T, Jarvis E, Nguyen L, Tang Q, MacKenzie TC. Direct and indirect antigen presentation lead to deletion of donor-specific T cells after in utero hematopoietic cell transplantation in mice. Blood. 2013;121:4595–4602. doi: 10.1182/blood-2012-10-463174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derderian SC, Togarrati PP, King C, Moradi PW, Reynaud D, Czechowicz A, Weissman IL, MacKenzie TC. In utero depletion of fetal hematopoietic stem cells improves engraftment after neonatal transplantation in mice. Blood. 2014;124:973–980. doi: 10.1182/blood-2014-02-550327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 19.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 20.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 23.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibboel D, Vermey-Keers C, Klug P, Gaillard JLJ, Koppenberg J, Molenaar JC. The natural history of gastroschisis during fetal life: development of the fibrous coating on the bowel loops. Teratology. 1986;33:267–272. doi: 10.1002/tera.1420330303. [DOI] [PubMed] [Google Scholar]
- 25.Bischoff SC, Mayer J, Nguyen QT, Stolte M, Manns MP. Immunohistological assessment of intestinal eosinophil activation in patients with eosinophilic gastroenteritis and inflammatory bowel disease. Am J Gastroenterol. 1999;94:3521–3529. doi: 10.1111/j.1572-0241.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- 26.Danzer E, Layne MD, Auber F, Shegu S, Kreiger P, Radu A, Volpe M, Adzick NS, Flake AW. Gastroschisis in mice lacking aortic carboxypeptidase-like protein is associated with a defect in neuromuscular development of the eviscerated intestine. Pediatr Res. 2010;68:23–28. doi: 10.1203/PDR.0b013e3181e17c75. [DOI] [PubMed] [Google Scholar]
- 27.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+ CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 28.Eberl G, Marmon S, Sunshine M-J, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2003;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 29.Bando JK, Liang H-E, Locksley RM. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol. 2014;16:153–160. doi: 10.1038/ni.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells -- a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 33.Van Gool F, Molofsky AB, Morar MM, Rosenzwajg M, Liang H-E, Klatzmann D, Locksley RM, Bluestone JA. Interleukin-5-producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood. 2014;124:3572–3576. doi: 10.1182/blood-2014-07-587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houben C, Davenport M, Ade-Ajayi N, Flack N, Patel S. Closing gastroschisis: diagnosis, management, and outcomes. J Pediatr Surg. 2009;44:343–347. doi: 10.1016/j.jpedsurg.2008.10.084. [DOI] [PubMed] [Google Scholar]
- 35.Kadry Z, Roux-Lombard P, Dayer JM, Lieberman J, Marks WH, Dafoe D, Cretin N, Cighetti G, Buhler L, Charbonnet P, Morel P. Impact of intestinal ischemia-reperfusion on cytokine profile and enterocyte viability in human syngeneic intestinal transplantation. Transplant. Proc. 2000;32:1292–1293. doi: 10.1016/s0041-1345(00)01231-8. [DOI] [PubMed] [Google Scholar]
- 36.Edgerton C, Crispín JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, Juang Y-T, Tsokos GC. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin. Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson MJ, Ke B, Shen X-D, Gao F, Busuttil RW, Kupiec-Weglinski JW, Farmer DG. Treatment with antithymocyte globulin ameliorates intestinal ischemia and reperfusion injury in mice. Surgery. 2012;152:843–850. doi: 10.1016/j.surg.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 39.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014;124:700–709. doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- 40.Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AK, Strominger JL, Brenner MB. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J-I, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2009;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 42.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie ANJ. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang K-M, Artis D. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hepworth MR, Monticelli LA, Fung TC, Ziegler CGK, Grunberg S, Sinha R, Mantegazza AR, Ma H-L, Crawford A, Angelosanto JM, Wherry EJ, Koni PA, Bushman FD, Elson CO, Eberl G, Artis D, Sonnenberg GF. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.la Cochetière, de M-F, Piloquet H, des Robert C, Darmaun D, Galmiche J-P, Rozé J-C. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of clostridium. Pediatr Res. 2004;56:366–370. doi: 10.1203/01.PDR.0000134251.45878.D5. [DOI] [PubMed] [Google Scholar]
- 48.Saitoh O, Kojima K, Sugi K, Matsuse R, Uchida K, Tabata K, Nakagawa K, Kayazawa M, Hirata I, Katsu K-I. Fecal eosinophil granule-derived proteins reflect disease activity in inflammatory bowel disease. Am J Gastroenterology. 1999;94:3513–3520. doi: 10.1111/j.1572-0241.1999.01640.x. [DOI] [PubMed] [Google Scholar]
- 49.Raab Y, Fredens K, Gerdin B, Hallgren R. Eosinophil activation in ulcerative colitis - studies on mucosal release and localization of eosinophil granule constituents. Dig Dis Sci. 1998;43:1061–1070. doi: 10.1023/a:1018843104511. [DOI] [PubMed] [Google Scholar]
- 50.Gerber BO, Zanni MP, Uguccioni M, Loetscher M, Mackay CR, Pichler WJ, Yawalkar N, Baggiolini M, Moser B. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol. 1997;7:836–843. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- 51.Smyth CM, Akasheh N, Woods S, Kay E, Morgan RK, Thornton MA, O'Grady A, Cummins R, Sheils O, Smyth P, Gleich GJ, Murray FM, Costello RW. Activated eosinophils in association with enteric nerves in inflammatory bowel disease. PLoS One. 2013;8:e64216–e64216. doi: 10.1371/journal.pone.0064216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiyosue M, Fujisawa M, Kinoshita K, Hori M, Ozaki H. Different susceptibilities of spontaneous rhythmicity and myogenic contractility to intestinal muscularis inflammation in the hapten-induced colitis. Neurogastroenterol Motil. 2006;18:1019–1030. doi: 10.1111/j.1365-2982.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 53.Midrio P, Vannucchi MG, Pieri L, Alaggio R, Faussone-Pellegrini MS. Delayed development of interstitial cells of Cajal in the ileum of a human case of gastroschisis. J Cell Mol Med. 2008;12:471–478. doi: 10.1111/j.1582-4934.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 55.Midrio P, Stefanutti G, Mussap M, D'Antona D, Zolpi E, Gamba P. Amnioexchange for fetuses with gastroschisis: is it effective? J Pediatr Surg. 2007;42:777–782. doi: 10.1016/j.jpedsurg.2006.12.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.