Summary
Enteropathogenic Escherichia coli (EPEC), enterohaemorrhagic E. coli (EHEC) and Citrobacter rodentium colonize their respective hosts while forming attaching and effacing lesions. Their infection strategy relies on translocation of a battery of type III secretion system effectors, including Map, EspM and EspT, which belong to the WxxxE/SopE family of guanine nucleotide exchange factors. Using the C. rodentium mouse model we found that EspT triggers expression of KC and TNFα in vivo. Indeed, a growing body of evidence suggests that, in addition to subversion of actin dynamics, the SopE and the WxxxE effectors activate signalling pathways involved in immune responses. In this study we found that EspT induces expression of the pro-inflammatory mediators cyclooxygenase-2 (COX-2) an enzyme involved in production of prostaglandin E(2) (PGE2), interleukin (Il)-8 and Il-1β in U937 human macrophages by activating the nuclear factor kappa-B (NF-κB), the extracellular signal-regulated kinases 1 and 2 (Erk1/2) and c-Jun N-terminal kinase (JNK) pathways. Since EspT modulates the activation of Cdc42 and Rac1, which mediates bacterial invasion into epithelial cells, we investigated the involvement of these Rho GTPases and bacterial invasion on pro-inflammatory responses and found that (i) Rac1, but not Cdc42, is involved in EspT-induced Il-8 and Il-1β secretion and (ii) cytochalasin D inhibits EspT-induced EPEC invasion into U937 but not Il-8 or Il-1β secretion. These results suggest that while EPEC translocates a number of effectors (i.e. NleC, NleD, NleE, NleH) that inhibit inflammation, a subset of strains, which encode EspT, employ an infection strategy that also involves upregulation of immune mediators.
Introduction
The human pathogens enteropathogenic Escherichia coli (EPEC) and enterohaemorrhagic E. coli (EHEC) and the mouse pathogen Citrobacter rodentium colonize the gut epithelium via attaching and effacing (A/E) lesions, which are characterized by localized effacement of the intestinal brush border microvilli, the rearrangement of host cytoskeletal proteins beneath the intimately attached bacteria and alteration of tight junctions (Spitz et al., 1995), which together contribute to the diarrhoeal disease. Similarly to other Gram-negative pathogens, EPEC, EHEC and C. rodentium encode a type III secretion system (T3SS), which is central to their infection strategy (Frankel et al., 1998). The T3SS translocates effector proteins directly from the bacteria to the eukaryotic cell cytoplasm, which target various cell-signalling pathways and subvert host cell responses (Wong et al., 2011).
The mucosal inflammation represents one of the main host defences against invading pathogens. Mucosal inflammation in the intestine is characterized by a distinct intestinal epithelial cell (IEC) response following the recognition of invading pathogens and subsequent infiltration of the mucosa with lymphocytes, in particular professional phagocytic cells including macrophages and neutrophils. Collectively, inflammation causes the upregulation of inflammatory genes, due to the activation of a number of transcription factors in both IECs and infiltrating leucocytes. Recently EPEC have been shown to use multiple strategies to downregulate IEC’s inflammation. T3SS effectors NleB, NleE, NleH, NleC and NleD attenuate pro-inflammatory neutrophilic chemokine CXCL-8 [also known as interleukin-8 (Il-8)] expression by blocking NF-κB or c-Jun N-terminal kinase (JNK) pathways (Gao et al., 2009; Nadler et al., 2010; Newton et al., 2010; Vossenkamper et al., 2010; Yen et al., 2010; Baruch et al., 2011; Pearson et al., 2011; Wan et al., 2011). Despite the immunosuppressive activity of multiple bacterial effectors, it is established that C. rodentium induces inflammatory responses in vivo characterized by an inflammatory cell infiltrate in the colon lamina propria and hyperplasia of the colonic crypts (Eckmann, 2006). This suggests that the immunosuppressive effectors may only partially block innate responses in vivo, and that host mechanisms (i.e. Toll-like receptors – TLRs – or Nod-like receptors – NLRs – that recognize pathogen-associated molecular patterns – PAMPs) offset their effect or that other translocated effectors can moderate and counterbalance the effect of the anti-inflammatory effectors.
Based on a conserved Trp–xxx–Glu motif, the T3SS effector proteins IpgB1 and IpgB2 of Shigella, SifA and SifB of Salmonella, and Map, EspM and EspT of EPEC and EHEC were grouped together into the WxxxE effectors family (Alto et al., 2006). Most of the WxxxE effectors, similarly to the Salmonella effector SopE, subvert actin dynamic by acting as guanine nucleotide exchange factors (GEF) of Rho GTPases (Bulgin et al., 2010). SopE, IpgB1 and EspT trigger membrane ruffles (Hardt et al., 1998; Ohya et al., 2005; Bulgin et al., 2009b), IpgB2 and EspM trigger stress fibres (Arbeloa et al., 2008) and Map trigger filopodia (Kenny et al., 2002) via activation of Rac1, RhoA and Cdc42 respectively. Despite the fact that WxxxE effectors and SopE are mainly involved in subversion of actin dynamics, a growing body of evidence suggests that these effectors are also implicated in activation of pathways involved in immune response. Indeed, by activating mitogen-activated protein kinases (MAPKs) and/or NF-κB pathway, SopE (Hobbie et al., 1997; Bruno et al., 2009; Muller et al., 2009), IpgB1 and IpgB2 (Fukazawa et al., 2008) induce innate immune responses.
To date, the involvement of EPEC effectors in the upregulation of the innate immune response has not been studied. To investigate the role of the WxxxE effectors Map, EspM2 and EspT on the inflammatory responses we used a human macrophage model of infection (U937), as this cell type is one of the first to respond to bacterial pathogens during infection. Here we report that EspT, which is implicated in EPEC cell invasion, also plays an important role in production of the immune mediators (Il-1β, Il-8 and PGE2) through mechanisms involving extracellular signal-regulated kinases (Erk), JNK and NF-κB.
Results
EspT induces expression of COX-2, Il-8 and Il-1β in U937 human macrophages
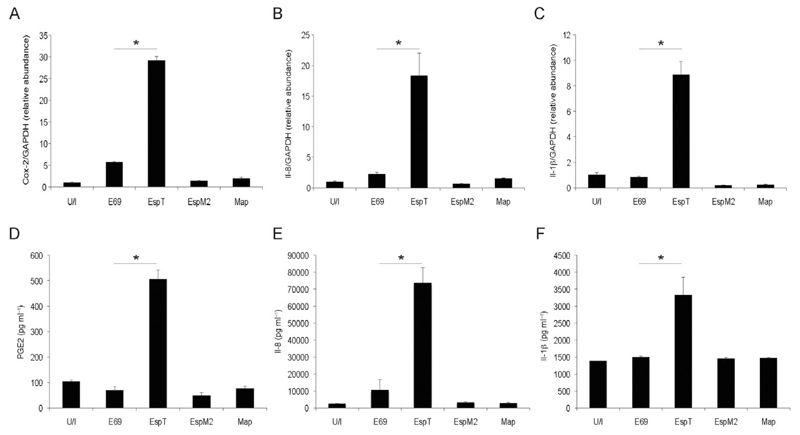
As the Shigella WxxxE effector IpgB1 triggers expression of Il-8 (Fukazawa et al., 2008) we investigated the impact of the IpgB1 EPEC homologue EspT and the other EPEC WxxxE effectors, Map and EspM2, on innate immune responses in macrophages. U937 macrophages were infected with either wild-type EPEC strain E2348/69 or E2348/69 expressing Map, EspM2 or EspT for 3 h then washed and incubated for a further 16 h in fresh media containing gentamicin to kill adherent bacteria. Compared with wild-type E2348/69, E2348/69 expressing EspT increased the level of cyclooxygenase-2 (COX-2) (Fig. 1A), Il-8 (Fig. 1B) and Il-1β (Fig. 1C) mRNA expression and in parallel the level of secreted prostaglandin E (2) (PGE2) (Fig. 1D), Il-8 (Fig. 1E) and Il-1β (Fig. 1F), while the transcript level of GAPDH control did not change (Fig. S1). In contrast expression of EspM2 or Map had no effect on gene expression or protein secretion of the proinflammatory mediators.
Fig. 1.
EspT upregulates production of pro-inflammatory mediators by macrophages. U937 cells were either left uninfected (U/I) or infected with EPEC E2348/69 (E69) or E2348/69 expressing EspT (EspT), EspM2 (EspM2) or Map (Map) for 3 h. Sixteen hours post infection, COX-2 (A), Il-8 (B) and Il-1β (C) mRNA expression and PGE2 (D), Il-8 (E) and Il-1β (F) secretion were determined. EPEC expressing EspT triggers expression and secretion of the analysed inflammatory mediators compare to wild-type EPEC, or EPEC expressing EspM2 or Map. Data are means + SD and are representative of at least three independent experiments; P value determined by Student’s T-test (*P < 0.05) comparing E69 with all other strains.
EspT induces Il-8 and Il-1β expressions through activation of NF-κB and Erk/JNK pathways
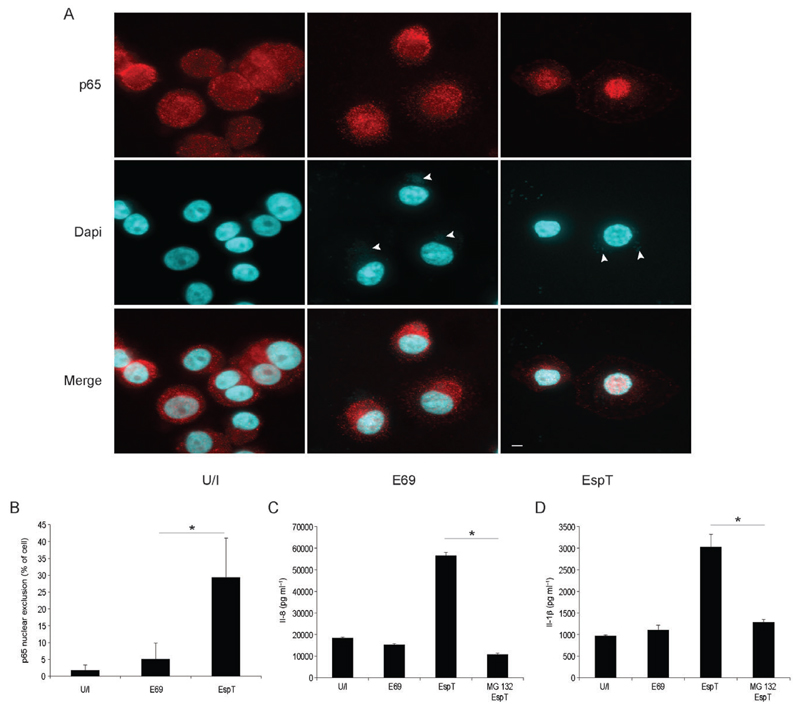
As NF-κB is a key transcription factor of inflammatory gene expression including COX-2 and Il-1β (Hoffmann et al., 2002), we examined its involvement in EspT-induced inflammatory responses in macrophages. U937 cells, infected either with E2348/69 expressing EspT or with wild-type E2348/69 as a control, were stained using antibodies directed against the NF-κB p65 subunit and Dapi (to stain nucleic acids). This has shown that in contrast to infection with wild-type E2348/69, E2348/69 expressing EspT induced targeting of p65 to the nucleus (Fig. 2A). Quantification of U937-infected cells revealed that 5 ± 4.7% and 29.3 ± 11.6% of adherent E2348/69 and E2348/69 expressing EspT respectively were associated with translocated p65 into the nucleus (Fig. 2B). Consistently, pre-incubation of cells with MG132, a proteasome inhibitor known to inhibit NF-κB activation by blocking the degradation of IκB, significantly reduced EspT-induced Il-1β (Fig. 2C) and Il-8 (Fig. 2D) secretion.
Fig. 2.
EspT induces Il-8 and Il-1β secretion via NF-κB activation. U937 cells were either left uninfected (U/I), or infected with E2348/69 (E69) or with E2348/69 expressing EspT (EspT). Two hours post infection p65 (red) was colocalized with Dapi (blue) in the nucleus specifically following infection with E2348/69 expressing EspT (A). Scale bar 5 µM. Quantification of infected cells with nuclear p65 staining (B). Pre-treatment of U937 cells with MG132 (MG132) 1 h before infection significantly reduces the level of EspT-mediated Il-8 (C) and Il-1β (D) secretion. Data are means + SD and are representative of at least two independent experiments; P value determined by Student’s T-test (*P < 0.05) comparing EspT strain with MG132/EspT condition.
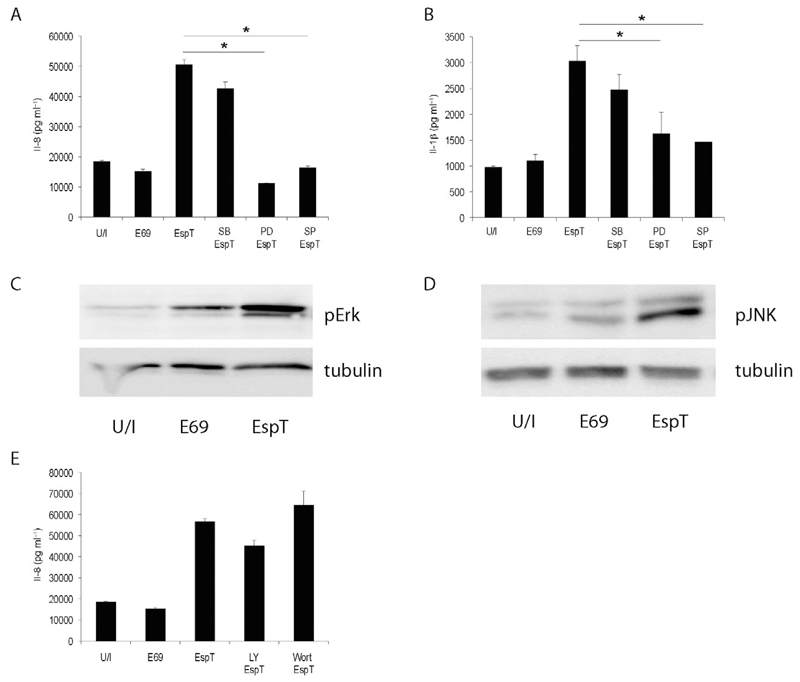
Since the MAPK cascade modulates innate immune responses (Hoffmann et al., 2002) we analysed the effect of EspT on MAPK activation. Using the p38, JNK and Erk inhibitors SB202190, SP600125 and PD98059, respectively, revealed that EspT induced Il-8 (Fig. 3A) and Il-1β (Fig. 3B) protein secretion via the Erk and JNK pathways but independently of p38 since only SP600125 and PD98059 significantly reduced the level of EspT-mediated Il-8 secretion. Western blotting has shown that EspT induced phosphorylation/activation of Erk (Fig. 3C) and JNK (Fig. 3D), but not of p38 (Fig. S2).
Fig. 3.
EspT induces Il-8 and Il-1β secretion via JNK and Erk activation. U937 cells were pre-treated with SB203580 (SB), PD98059 (PD) or SP600125 (SP) 1 h before infection. PD and SP significantly reduce the level of EspT-mediated Il-8 (A) and Il-1β (B) secretion. Western blot of whole-cell lysates probed with antibodies directed against phospho-Erk (pErk) (C) and phospho-JNK (pJNK) (D) and normalized using a-tubulin antibody (tubulin) show that EPEC expressing EspT triggers phosphorylation of both Erk and JNK MAPKs. Pre-treatment with Wortmannin (Wort) or LY294002 (LY) 1 h before infection shows no significant changes on the level of EspT-induced Il-8 secretion (E). Data are means + SD and are representative of at least three independent experiments; P value determined by Student’s T-test (*P < 0.05) comparing EspT strain with all other conditions as indicated.
As the PI3K/AKT pathway is also involved in immune response (Arbibe et al., 2000), we analysed the effect of PI3K inhibitors LY294002 and Wortmannin on EspT-mediated Il-8 secretion. This revealed that infection of U937 cells with E2348/69 expressing EspT in the presence or absence of either LY294002 or Wortmannin resulted in secretion of Il-8 at comparable levels (Fig. 3E).
EspT induces inflammatory response independently of bacterial uptake
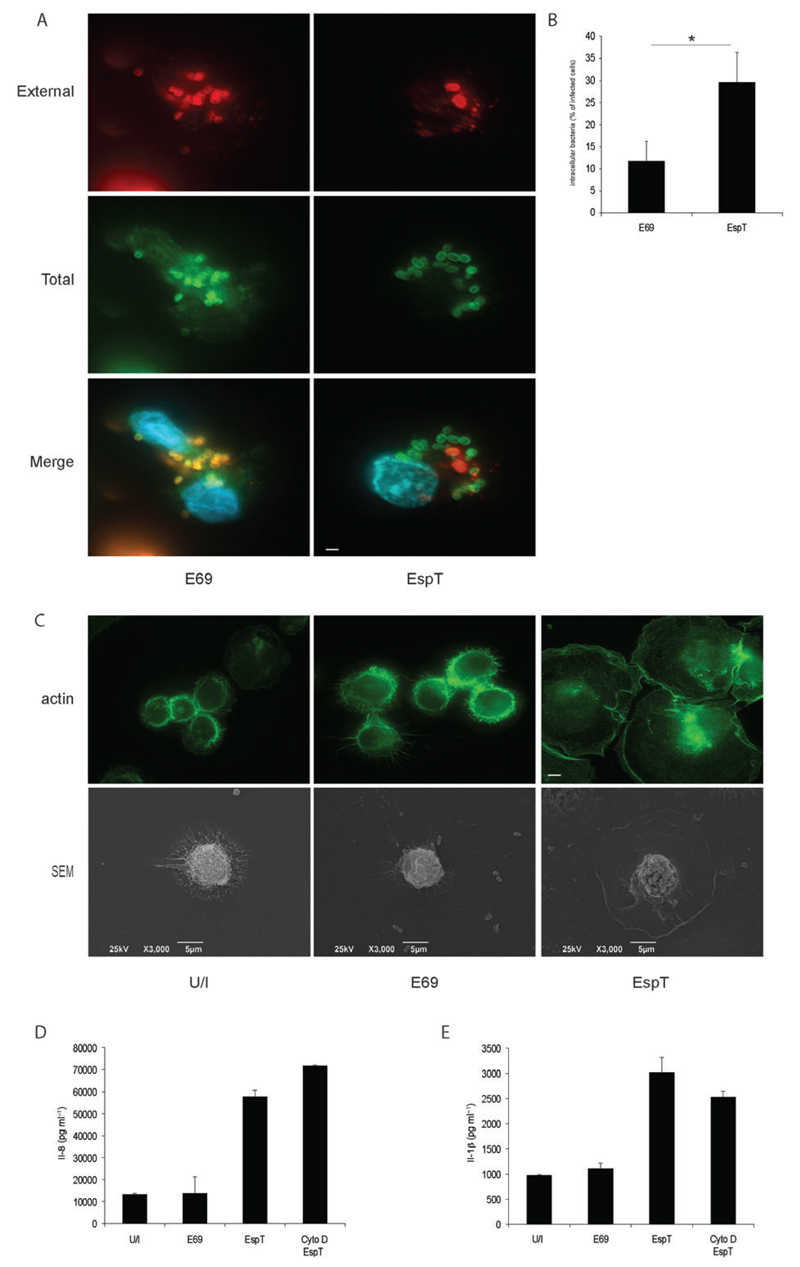
While various EPEC effectors are able to block phagocytosis of non-opsonized bacteria by macrophages (e.g. EspF and EspH) (Marches et al., 2008; Dong et al., 2010), we have recently reported that EspT can mediate invasion of EPEC into non-phagocytic cell (Bulgin et al., 2009a). Thus, we analysed whether EPEC expressing EspT can invade macrophages and whether bacterial uptake is required for induction of the inflammatory mediators. We observed by immunostaining that E2348/69 expressing EspT invaded U937 better (29.6 ± 6.7%) than E2348/69 alone (11.8 ± 4.4%) (Fig. 4A and B), a process that was associated with actin remodelling and cell spreading as observed by immunostaining and scanning electron microscopy (Fig. 4C). As expected, we found that cytochalasin D, a well-characterized inhibitor of actin polymerization and hence bacterial invasion/phagocytosis, blocked EPEC internalization and macrophage cell spreading (data not shown). However cytochalasin D did not prevent EspT-induced Il-8 (Fig. 4D) and Il-1β (Fig. 4E) secretions, indicating that EspT translocation, rather than microfilament reorganization or bacterial internalization, is involved in the observed inflammatory response.
Fig. 4.
EspT induces Il-8 and Il-1β secretions independently of bacterial uptake.
A and B. U937 cells were either uninfected (U/I), or infected with the E2348/69 (E69) or E2348/69 expressing EspT (EspT). After 3 h cells were washed and the number of intracellular and extracellular bacteria, quantified by immunofluorescence using O127 antiserum (red – external, green – total) and Dapi (blue), revealed that EspT triggers EPEC internalization into macrophages.
C. Immunofluorescence using AlexaFluor 488 phalloidin (green – upper section) and scanning electron microscopy (SEM – lower section) show that EspT triggers macrophage cell spreading. Scale bar 5 µm.
D and E. Pre-treatment of U937 with cytochalasin D (Cyto D) for 1 h before infection inhibited cell spreading but showed no significant reduction in EspT-mediated Il-8 (D) and Il-1β (E) secretions. Data are means + SD and are representative of at least three independent experiments.
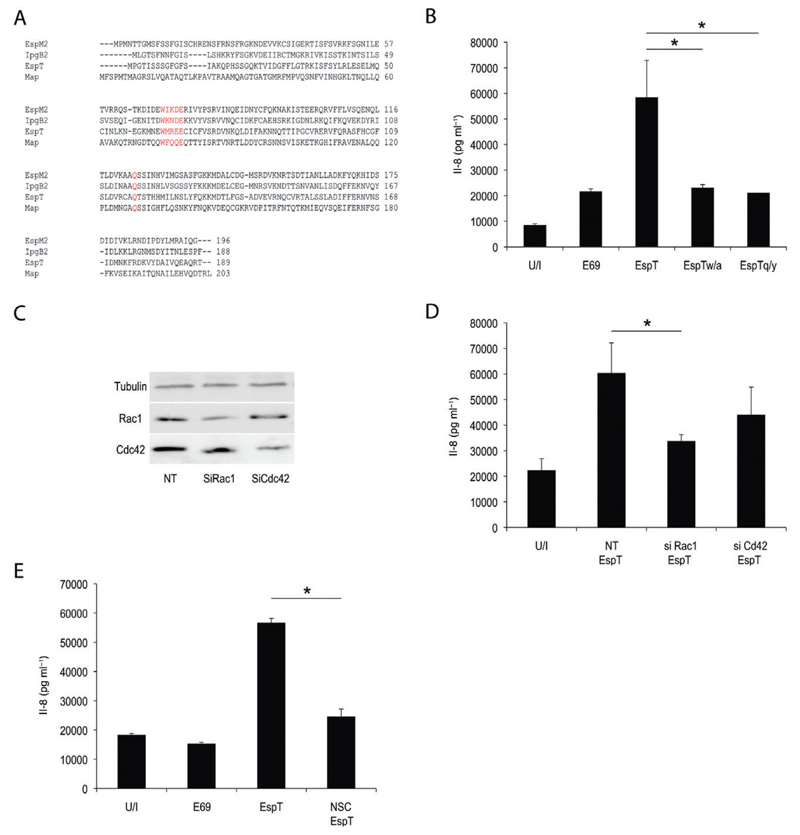
EspT-mediated Il-8 production is regulated via RAC1
Subversion of actin dynamics by Map, IpgB1, IpgB2, EspM2, EspM3 and EspT is dependent on their invariant structural WxxxE motif (Alto et al., 2006). More recently, the active site of the WxxxE effectors has been described (Arbeloa et al., 2010). Using sequence alignment we identified the putative active site of EspT (Fig. 5A). In order to determine whether the WxxxE motif or the putative active site are required for EspT-induced pro-inflammatory mediator release, residue W63 from the WxxxE site and the Q117 from the putative active site were substituted by A and Y respectively (Fig. 5A), mutations that were already shown to drastically reduce the activity of WxxxE effectors (Huang et al., 2009; Arbeloa et al., 2010). U937 cells were then infected with E2348/69 expressing EspTWT, EspTW63A or EspTQ117Y; infection with wild-type E2348/69 was used as a control. We found that EPEC expressing EspTW63A and EspTQ117Y triggered significantly lower level of Il-8 secretion compared with E2348/69 expressing EspTWT (Fig. 5B). Since EspT was shown to specifically activate Rac1 and Cdc42, we investigated their involvement in EspT-induced secretion of pro-inflammatory mediators. We found that EspT-mediated Il-8 production in U937 was significantly reduced following depletion of Rac1 (Fig. 5D), but not Cdc42, by siRNA (Fig. 5D). We used a pharmacological approach to confirm that Rac1 is required for EspT-mediated Il-8 production. Towards this end U937 cells were treated with NSC23766, a Rac1-specific inhibitor interfering with its interaction with Rac-specific GEFs. This has shown that NSC23766 prevented EspT-induced Il-8 secretion (Fig. 5E). Thus, the observed inflammatory activation in macrophages by bacteria expressing EspT is independent of cellular internalization but dependent on activation of Rac1.
Fig. 5.
EspT-mediated Il-8 production is regulated via RAC1.
A. Alignment of IpgB1, Map EspM2 and EspT effector proteins; the point mutations at position W63 and Q117 are indicated in red.
B. U937 cells were infected with E2348/69 or E2348/69 expressing EspTWT, EspTW/A or EspTQ/Y. EspTW/A and EspTQ/Y mutants drastically reduced the level of EspT-mediated Il-8 secretion.
C and D. U937 cells were transfected with non-targeting (NT) siRNA or siRNA targeted against Rac1 (SiRac1) or Cdc42 (SiCdc42) for 48 h. Successful knock-down was confirmed by Western blotting (C). Transfected U937 cells were either uninfected (U/I) or infected with E2348/69 expressing EspT (EspT). siRac1 inhibits the level of EspT-mediated Il-8 secretion while siCdc42 showed no effect on the level of Il-8 secretion.
E. Pre-treatment with NSC23766 (NSC) for 1 h reduced the level of EspT-mediated Il-8 secretion. Data are means + SD and are representative of at least three independent experiments; P value determined by Student’s T-test (*P < 0.05) comparing EspT strain with all other conditions as indicated.
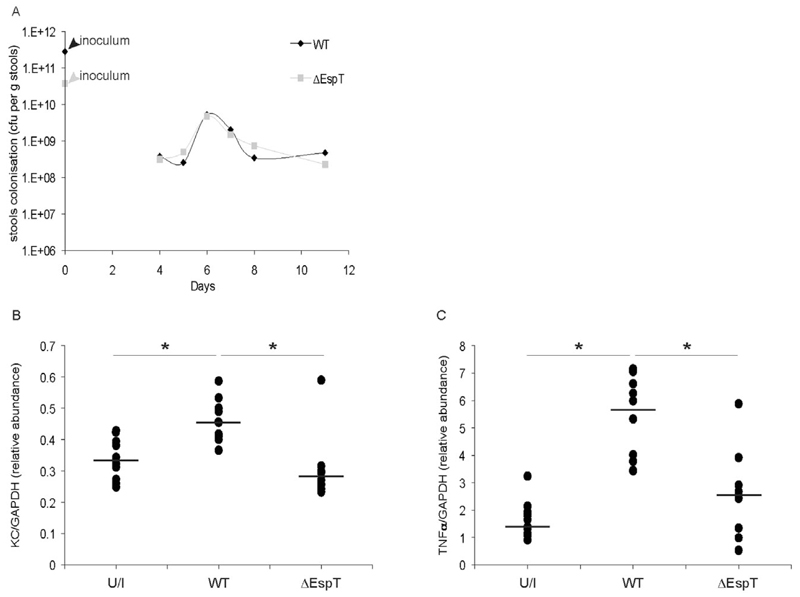
EspT induces expression of KC and TNFα in vivo
As C. rodentium induces mucosal inflammation in mice (Hirata et al., 2010), we determined the relevance of the in vitro EspT function on induction of immune mediator in vivo. Towards this end we infected C57/BL-6 mice with wild-type C. rodentium and an isogenic espT mutant. Wild-type and the mutant C. rodentium strains colonized the mouse intestine at comparable levels (Fig. 6A). Importantly, while we did not detect expression of the proinflammatory cytokine Il-1β following infection with either strain, the espT mutant triggered significantly reduced levels of KC, the murine equivalent of Il-8 (Fig. 6B) and TNFα (Fig. 6C) mRNA compared with infection with the parental, wild-type strain.
Fig. 6.
EspT induces mucosal expression of KC and TNFα. C57BI/6 mice were orally inoculated with PBS (PBS), wild-type C. rodentium (WT) or C. rodentium espT mutant (ΔespT).
A. Colonization and clearance dynamics of C. rodentium strains shows the wild-type and mutant strains colonized at comparable level over a 12-day period.
B and C. At day 12, sections of colon were collected and KC (B) and TNFα (C) mRNA expressions were analysed and normalized with mRNA expression of GAPDH. Wild-type C. rodentium expressing EspT triggers significantly higher levels of KC and TNFα mRNA compared with C. rodentium ΔespT. Data are means + SD and are representative of two independent experiments; P value determined by Mann–Whitney U-test (*P < 0.05) comparing wild-type with uninfected and ΔespT.
Discussion
Enteropathogenic E. coli, EHEC and C. rodentium utilize their T3SS to inject effector proteins into the mammalian cell in order to manipulate cell-signalling pathways and subvert host cell responses. The WxxxE effector EspT has recently been shown to trigger bacterial invasion into epithelial cell (Bulgin et al., 2009a). Here we demonstrated for the first time that EspT mediates internalization of EPEC into macrophages, suggesting that it overrides the anti-phagocytic activity of EspF (Marches et al., 2008) and EspH (Dong et al., 2010).
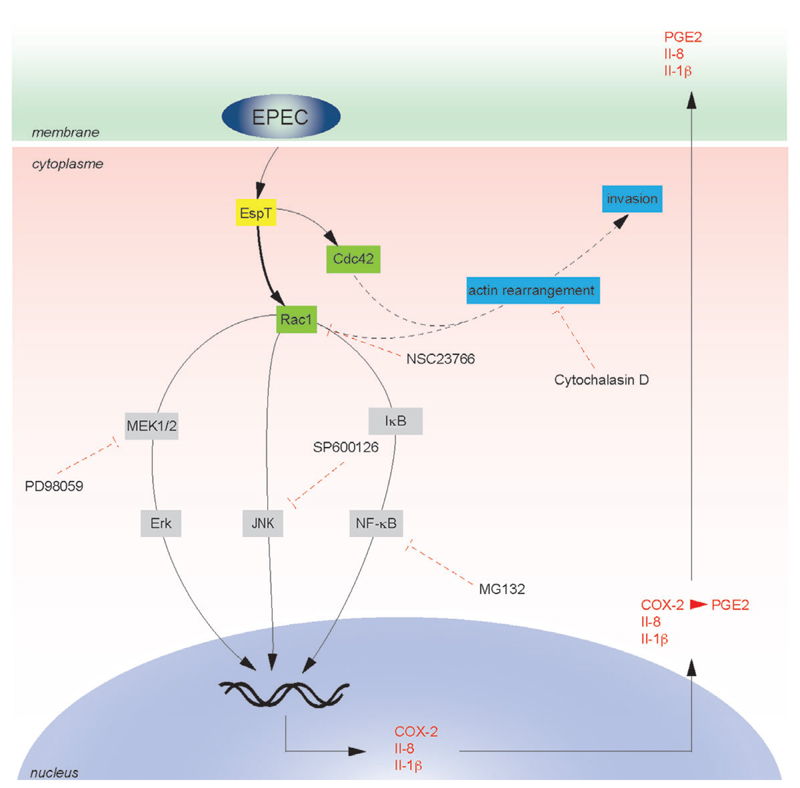
Toll-like receptors and NLRs are the key host receptors involved in the recognition of microbial PAMPs (e.g. TLR4 and TLR5 which recognize LPS and flagelin respectively) leading to production of inflammatory mediators through various adaptors (e.g. MyD88, IRAK, TRAF6 or RIP2) (Medzhitov, 2001). However, a number of studies have recently shown that bacterial effectors involved in cell invasion are also able to induce inflammatory response. For example, Fukazawa et al. (2008) showed that induction of NF-κB activation and initiation of innate immune responses by the Shigella WxxxE effector IpgB2 is dependent on activation of RhoA, RIP2 and ROCK pathways (Fukazawa et al., 2008). Invasion of epithelial cells by Salmonella stimulates innate immune responses by mechanisms that do not involve TLRs or NLRs but rather the bacterial effector proteins SopE, SopE2 and SopB (Hobbie et al., 1997; Bruno et al., 2009). Yersinia invasion into epithelial cells activates the NF-κB pathway and Il-8 expression in a mechanism involving invasin-mediated activation of Rac1 (Grassl et al., 2003). In this study we identified EspT as an effector of A/E pathogens that can trigger inflammatory response in vivo. Epithelial cells, macrophages and dendritic cells represent the first sentinel cells implicated in the recognition of invading pathogens leading to the induction of inflammatory response. Since we did not record any significant EspT-induced cytokine production using epithelial cells (data not shown), we investigated its impact on macrophages. We found that EspT, but not the EPEC WxxxE effectors Map and EspM2, induces production of Il-8, Il-1β and PGE2 by U937 macrophages. Il-8 and Il-1β are important mediators of inflammatory responses. PGE2, which is one of the most abundant metabolites of arachidonic acid generated through an enzymatic cascade controlled by COX-1 and COX-2, also plays a role in inflammatory response by increasing vascular permeability, fever generation and hyperalgesia (Trebino et al., 2003) Salmonella has already been shown to induce COX-2 expression in macrophages (Uchiya et al., 2004; Cristina Cerquetti et al., 2008). Importantly, in our study, induction of the proinflammatory responses by EspT occurred independently of bacterial invasion. Mutagenesis of the putative EspT active loop residue Q117 (Huang et al., 2009) and the structural residue W63 (Alto et al., 2006; Arbeloa et al., 2010) severely attenuated the ability of EspT to trigger production of Il-8 suggesting that activation of Rho GTPase by EspT is involved in Il-8 production. Moreover, we found using siRNA and specific inhibitors that Rac1, but not Cdc42, is involved in EspT-induced Il-8 production. Rac1 regulates cell signalling by a variety of downstream effectors including p21-activated kinases (PAKs) (Naumann et al., 1998) and Phosphatidylinositol 3-kinases (PI3K) (Arbibe et al., 2000), which are required for MAPKs and NF-κB activation. By using the PAK inhibitor IPA3 and the PI3K inhibitors LY294002 and Wortmannin we found that these pathways are not involved in EspT-induced Il-8 production in the U937 cells. In contrast, we found that EspT-induced Il-8 and Il-1β secretion by U937 cells is dependent on NF-κB signalling and involves MAPK, Erk and JNK (Fig. 7). The JNK and Erk inhibition did not affect NF-κB nuclear translocation (data not shown). In addition, the global caspase inhibitor z-VAD-FMK, known to block caspases including caspase-1 which process pro-Il-1β into the mature secreted form Il-1β, did not affect EspT-induced secretion of Il-1β (data not shown).
Fig. 7.
A model deciphering the signalling pathway hijacked by EspT leading to induction of inflammatory responses in macrophages. Translocated EspT activates Rac1 and Cdc42 leading to bacterial internalization in a microfilament (black dash lines)-dependent manner. Activation of Rac1 upregulates COX-2, Il-8 and Il-1β genes expression and the secretions of PGE2 and Il-1β through Erk, JNK and NF-κB pathways. The dash red lines represent the targets of the specific inhibitors used in this study. EspT appears to antagonize the inhibitory impact of immunosuppressive effectors translocated by EPEC.
Most of the recent studies of EPEC effectors interfering with inflammatory response have been performed using epithelial cells. When testing the function of EspT in HeLa, Caco-2 and Beas-2B cell we did not observe any significant EspT-induced Il-8 production. However, we found that EspT also triggered Il-8 production in human THP-1 macrophages. These results suggest that while the function of EspT in the context of epithelial cells is more likely limited to cell invasion, it triggers internalization, cell spreading and expression of pro-inflammatory responses in macrophages.
Many EPEC effectors have been shown to inhibit immune responses. Ruchaud-Sparagano et al. (2007) have shown that wild-type EPEC suppresses the ability of Caco-2 cells to activate cell signalling leading to secretion of Il-8 in a T3SS-dependent manner (Ruchaud-Sparagano et al., 2007). More recently, NleB, NleE, NleH, NleC and NleD have been shown to inhibit Il-8 production in epithelial cells by targeting different stages of the inflammatory response (Gao et al., 2009; Nadler et al., 2010; Newton et al., 2010; Vossenkamper et al., 2010; Yen et al., 2010; Baruch et al., 2011; Pearson et al., 2011; Wan et al., 2011). Our finding that EspT activates immune responses is therefore intriguing, although not surprising considering that translocation of effectors of antagonistic functions is becoming a common theme in bacterial pathogenesis. Indeed, Salmonella (Collier-Hyams et al., 2002; Le Negrate et al., 2008) and Shigella (Arbibe et al., 2007; Ashida et al., 2010) secrete multiple T3SS effectors that interfere with inflammatory signalling and WxxxE/SopE effectors that induce pro-inflammatory responses (Fukazawa et al., 2008; Bruno et al., 2009). Our data show that overexpression of EspT overrides both the antiphagocytic activity exhibited by EspF and EspH and the immunosuppressive action of NleB, NleE, NleH, NleC and NleD.
Although we have shown that EspT is involved in induction of inflammatory responses during C. rodentium infection, espT is not found in EHEC and is only rarely found in human EPEC strains (Arbeloa et al., 2009). Importantly, the espT positive EPEC strain E110019 was responsible for a severe diarrhoeal outbreak in Finland in 1987, which uniquely affected children and adults alike (Viljanen et al., 1990). As EspT can affect the balance between pro and anti-inflammatory responses we suspect this may contribute, at least in part, to virulence. Indeed, our in vivo study has shown that although C. rodentium expresses all the known immunosuppressive effectors it induces a mild inflammatory response in vivo in a mechanism involving EspT. These results add to the growing recognition that bacterial pathogens use T3SS effectors to optimize their colonization niche by affecting the resident microbiota (Stecher et al., 2007; Sekirov et al., 2010) via mechanisms involving redundant, synergistic or antagonistic activities.
Experimental procedures
Bacterial strains and growth conditions
Escherichia coli strains and plasmids used in this study are listed in Table S1 (Levine et al., 1978; Schlosser-Silverman et al., 2000; Wiles et al., 2004; Simpson et al., 2006). Bacteria were cultured in Luria–Bertani (LB) medium or in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with ampicillin (100 µg ml−1) or kanamycin (50 µg ml−1) and nalidixic acid (50 µg ml−1) as required.
Cell culture and infection
U937 human promyeloid cells were obtained from American Type Culture Collection (Rockville, MD). The cells were grown in RPMI 1640 medium (Invitrogen, Green Island, NY) supplemented with 10% heat-inactivated FBS (Invitrogen), 2 mM L-glutamine at 37°C in a 5% CO2 humidified atmosphere as described. U937 cells were differentiated 24-well plates into macrophage-like cells by incubation with 15 nM PMA (Calbiochem, San Diego, CA) for 48 h. One hour before infection, the cells were washed in phosphate buffer (PBS), and incubated with 500 µl of fresh DMEM without FBS and supplemented or not with pharmacological inhibitors as required. After 1 h 500 µl of the primed and IPTG (1 µM final)-induced bacteria were added to each well and the infection assays were carried out at 37°C in 5% CO2. After 1–3 h, the cells were then washed three times in PBS and processed or incubated for 16 h with fresh DMEM without FBS supplement with 200 µg ml−1 gentamicin.
Site-directed mutagenesis
Site-directed mutagenesis was carried out using a Quickchange II kit (Stratagene) according to the manufacturer’s instructions. Primers listed in Table S1 were designed using the Quickchange mutagenic primer design program (Stratagene). Plasmids pSA10 containing espT was used as templates for the mutagenic reactions. Colonies were screened by sequencing and alignment to wild-type sequences to confirm mutagenesis.
Scanning electron microscopy
Glass coverslips were seeded with cells and infected for 2 h with the appropriate strains. The cells were washed three times in PBS pH 7.2 and then fixed with 2.5% gluteraldehyde (Sigma) in phosphate buffer pH 7.2 for 15 min. The coverslips were then washed in phosphate buffer pH 7.2 three times before being post-fixed in 1% Osmium Tetroxide for 1 h. The cells were then washed three times in phosphate buffer before being washed for 15 min in graded ethanol solutions from 50% to 100% to dehydrate the samples. The cells were then transferred to an Emitech K850 Critical Point drier and processed according to the manufacturer’s instructions. The coverslips were coated in gold/palladium mix using an Emitech Sc7620 minisputter to a thickness of approximately 370A°. Samples for scanning electron microscopy (SEM) were then examined blindly at an accelerating voltage of 25 kV using a Jeol JSM-6390.
Immunofluorescence staining and microscopy
Glass coverslips were seeded with cells and infected for 2 h with the appropriate strains. The cells were washed three times in PBS and then fixed with 3% paraformaldehyde (PFA) for 15 min before washing three more times in PBS. After quenching for 10 min with 50 mM NH4Cl and permeabilized for 4 min in PBS 0.2% Triton X-100, cells were then washed three times in PBS and blocked for 1 h with PBS 5% BSA before incubation with primary and secondary antibodies. The primary antibody rabbit anti-NF-κB p65 subunit (SantaCruz Biotechnology) was used at a dilution of 1:100, while rabbit anti-O127 was used at a dilution of 1:500. Coverslips were incubated with the primary antibody for 1 h, washed three times in PBS and incubated with the secondary antibodies. Donkey anti-rabbit IgG conjugated to a Cy2 or Cy3 fluorophore (Jackson laboratories) were used at a 1:200. Actin was stained using AlexaFluor 647 phalloidin or Oregon Green phalloidin (Invitrogen) at a 1:100 dilution. All dilutions were in PBS 5% BSA. Coverslips were mounted on slides using Pro-LongH Gold antifade reagent (Invitrogen) and visualized by Zeiss Axioimager immunofluorescence microscope using the following excitation wavelengths: Cy3 – 550 nm, Cy5 – 650 nm and Oregon Green – 488 nm. All images were analysed using the Axiovision Rel 4.5 software.
RNA extraction and real-time reverse-transcription PCR analysis
After 3 h infection, the cells were then washed three times in PBS and incubated 16 h with fresh DMEM without FBS supplement with 200 µg ml−1 gentamicin. Regarding the in vivo assay, at day 12 post inoculum, the mice were culled and the colonic tissues were collected and preserved in RNAlater RNA Stabilization Reagent (Qiagen). Then, total RNA was extracted using an RNeasy kit from Qiagen. DNase treatment was performed using 2 µg of extracted RNA, 1 µl of DNase I (Amersham Biosciences) and 0.5 µl of RNasin (Promega) in a total volume of 20 µl in the manufacturer’s buffer. cDNA were obtained by incubating RNA with 1 mM dNTP (Promega), 1.5 µl of hexamers as primers, 20 units of RNasin (Promega), and 300 units of Moloney murine leukaemia virus reverse transcriptase RNase H minus (Promega) in a total volume of 50 µl of the manufacturer’s buffer for 1 h at 42°C and 10 min at 70°C. Real-time PCR was performed using the SYBR Green kit (Stratagene Brilliant II) and analysed using the MxPro software (Stratagene). The primers listed in Table S1 were used.
Cytokines and PGE2 enzyme immunoassays
After 3 h infection, the cells were then washed three times in PBS and incubated 16 h with fresh DMEM without FBS supplement with 200 µg ml−1 gentamicin. Then the supernatants were collected and PGE2 concentration was measured using Enzyme Immunoassay (EIA) kit (Cayman chemical). Il-8, Il-1β concentrations were measured using DuoSet ELISA kits from R&D systems.
Protein extraction and Western blot analyses
After 1 h infection, proteins were extracted from U937 cells using RIPA supplement with anti-proteases (Roche) and anti-phosphatases (Roche). Five micrograms of total proteins were subjected to SDS-PAGE. Proteins were transferred to membrane from Millipore, which were blocked with 5% BSA and probed overnight at 4°C with the indicated antibodies (Erk, phospho Erk, JNK, phospho JNK – cell signalling – and tubulin – Sigma). After washing with TBS tween, the immunoreactive bands were visualized with a specific peroxidase-conjugated anti-rabbit or anti-mousse IgG antibody (Jackson laboratories) and using an ECL Plus Western Blotting Detecting System (Amersham, Biosciences).
Mice
Pathogen-free 18–20 g of C57Bl/6 mice were purchased from Charles River. All animals were housed in individually HEPA-filtered cages with sterile bedding and free access to sterilized food and water. All animal experiments were performed in accordance with the Animals Scientific Procedures (Act 1986) and were approved by the local Ethical Review Committee. Independent single infection experiments were performed three times using three to six mice per group. Mice inoculated with wild-type strain and uninfected mice were included in parallel with mutant strains by oral gavage with 200 µl of overnight LB-grown C. rodentium suspension in PBS (1·1011cfu). The number of viable bacteria used as inoculum was determined by retrospective plating onto LB agar containing antibiotics. Stool samples were recovered aseptically at various time points after inoculation and the number of viable bacteria per gram of stool was determined by plating onto LB agar (Wiles et al., 2004). At day 12 post inoculum, the mice were culled and segments of the terminal colon of each mouse were collected for mRNA studies as described above.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. The strains used do not affect the levels of GAPDH transcript in U937 cells. U937 cells were either left uninfected (U/I) or infected with EPEC E2348/69 (E69) or E2348/69 expressing EspT (EspT), EspM2 (Esp1M2) or Map (Map) for 3 h. Sixteen hours post infection, GAPDH mRNA expression was analysed either using a quantitative (A) or using a semiquantitative (B) RT-PCR assay. Data are means + SD and are representative of three independent experiments.
Fig. S2. p38 phosphorylation is unaltered in the presence of EspT. Western blot of whole-cell lysates probed with antibodies directed against phospho-P38 (pP38) and normalized using total P38 antibody (P38) show that EPEC expressing EspT triggers the same level of phosphorylation of P38 over the time.
Table S1. Strains, plasmids and primers used in this study.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Acknowledgements
We warmly thank Dr Michael Bright and Dr Ana Arbeloa for their advice and technical supports. This study was supported by grants from the BBSRC and the Wellcome Trust.
References
- Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 2006;124:133–145. doi: 10.1016/j.cell.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Arbeloa A, Bulgin RR, MacKenzie G, Shaw RK, Pallen MJ, Crepin VF, et al. Subversion of actin dynamics by EspM effectors of attaching and effacing bacterial pathogens. Cell Microbiol. 2008;10:1429–1441. doi: 10.1111/j.1462-5822.2008.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeloa A, Blanco M, Moreira FC, Bulgin R, Lopez C, Dahbi G, et al. Distribution of espM and espT among enteropathogenic and enterohaemorrhagic Escherichia coli. J Med Microbiol. 2009;58:988–995. doi: 10.1099/jmm.0.010231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeloa A, Garnett J, Lillington J, Bulgin RR, Berger CN, Lea SM, et al. EspM2 is a RhoA guanine nucleotide exchange factor. Cell Microbiol. 2010;12:654–664. doi: 10.1111/j.1462-5822.2009.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- Arbibe L, Kim DW, Batsche E, Pedron T, Mateescu B, Muchardt C, et al. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat Immunol. 2007;8:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- Ashida H, Kim M, Schmidt-Supprian M, Ma A, Ogawa M, Sasakawa C. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat Cell Biol. 2010;12:66–73. doi: 10.1038/ncb2006. sup 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Gur-Arie L, Nadler C, Koby S, Yerushalmi G, Ben-Neriah Y, et al. Metalloprotease type III effectors that specifically cleave JNK and NF-kappaB. EMBO J. 2011;30:221–231. doi: 10.1038/emboj.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno VM, Hannemann S, Lara-Tejero M, Flavell RA, Kleinstein SH, Galan JE. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 2009;5:e1000538. doi: 10.1371/journal.ppat.1000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgin R, Arbeloa A, Goulding D, Dougan G, Crepin VF, Raymond B, Frankel G. The T3SS effector EspT defines a new category of invasive enteropathogenic E. coli (EPEC) which form intracellular actin pedestals. PLoS Pathog. 2009a;5:e1000683. doi: 10.1371/journal.ppat.1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgin RR, Arbeloa A, Chung JC, Frankel G. EspT triggers formation of lamellipodia and membrane ruffles through activation of Rac-1 and Cdc42. Cell Microbiol. 2009b;11:217–229. doi: 10.1111/j.1462-5822.2008.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgin R, Raymond B, Garnett JA, Frankel G, Crepin VF, Berger CN, Arbeloa A. Bacterial guanine nucleotide exchange factors SopE-like and WxxxE effectors. Infect Immun. 2010;78:1417–1425. doi: 10.1128/IAI.01250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, et al. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- Cristina Cerquetti M, Hovsepian E, Sarnacki SH, Goren NB. Salmonella enterica serovar Enteritidis dam mutant induces low NOS-2 and COX-2 expression in macrophages via attenuation of MAPK and NF-kappaB pathways. Microbes Infect. 2008;10:1431–1439. doi: 10.1016/j.micinf.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Dong N, Liu L, Shao F. A bacterial effector targets host DH-PH domain RhoGEFs and antagonizes macrophage phagocytosis. EMBO J. 2010;29:1363–1376. doi: 10.1038/emboj.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann N Y Acad Sci. 2006;1072:28–38. doi: 10.1196/annals.1326.008. [DOI] [PubMed] [Google Scholar]
- Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- Fukazawa A, Alonso C, Kurachi K, Gupta S, Lesser CF, McCormick BA, Reinecker HC. GEF-H1 mediated control of NOD1 dependent NF-kappaB activation by Shigella effectors. PLoS Pathog. 2008;4:e1000228. doi: 10.1371/journal.ppat.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wan F, Mateo K, Callegari E, Wang D, Deng W, et al. Bacterial effector binding to ribosomal protein s3 subverts NF-kappaB function. PLoS Pathog. 2009;5:e1000708. doi: 10.1371/journal.ppat.1000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassl GA, Kracht M, Wiedemann A, Hoffmann E, Aepfelbacher M, von Eichel-Streiber C, et al. Activation of NF-kappaB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell Microbiol. 2003;5:957–971. doi: 10.1046/j.1462-5822.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galan JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Egea L, Dann SM, Eckmann L, Kagnoff MF. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe. 2010;7:151–163. doi: 10.1016/j.chom.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie S, Chen LM, Davis RJ, Galan JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Huang Z, Sutton SE, Wallenfang AJ, Orchard RC, Wu X, Feng Y, et al. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat Struct Mol Biol. 2009;16:853–860. doi: 10.1038/nsmb.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny B, Ellis S, Leard AD, Warawa J, Mellor H, Jepson MA. Co-ordinate regulation of distinct host cell signalling pathways by multifunctional enteropathogenic Escherichia coli effector molecules. Mol Microbiol. 2002;44:1095–1107. doi: 10.1046/j.1365-2958.2002.02952.x. [DOI] [PubMed] [Google Scholar]
- Le Negrate G, Faustin B, Welsh K, Loeffler M, Krajewska M, Hasegawa P, et al. Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-kappaB, suppresses IkappaBalpha ubiquitination and modulates innate immune responses. J Immunol. 2008;180:5045–5056. doi: 10.4049/jimmunol.180.7.5045. [DOI] [PubMed] [Google Scholar]
- Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;1:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Marches O, Covarelli V, Dahan S, Cougoule C, Bhatta P, Frankel G, Caron E. EspJ of enteropathogenic and enterohaemorrhagic Escherichia coli inhibits opsono-phagocytosis. Cell Microbiol. 2008;10:1104–1115. doi: 10.1111/j.1462-5822.2007.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Muller AJ, Hoffmann C, Galle M, Van Den Broeke A, Heikenwalder M, Falter L, et al. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe. 2009;6:125–136. doi: 10.1016/j.chom.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Nadler C, Baruch K, Kobi S, Mills E, Haviv G, Farago M, et al. The type III secretion effector NleE inhibits NF-kappaB activation. PLoS Pathog. 2010;6:e1000743. doi: 10.1371/journal.ppat.1000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M, Rudel T, Wieland B, Bartsch C, Meyer TF. Coordinate activation of activator protein 1 and inflammatory cytokines in response to Neisseria gonorrhoeae epithelial cell contact involves stress response kinases. J Exp Med. 1998;188:1277–1286. doi: 10.1084/jem.188.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton HJ, Pearson JS, Badea L, Kelly M, Lucas M, Holloway G, et al. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-kappaB p65. PLoS Pathog. 2010;6:e1000898. doi: 10.1371/journal.ppat.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya K, Handa Y, Ogawa M, Suzuki M, Sasakawa C. IpgB1 is a novel Shigella effector protein involved in bacterial invasion of host cells. Its activity to promote membrane ruffling via Rac1 and Cdc42 activation. J Biol Chem. 2005;280:24022–24034. doi: 10.1074/jbc.M502509200. [DOI] [PubMed] [Google Scholar]
- Pearson JS, Riedmaier P, Marches O, Frankel G, Hartland EL. A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-kappaB for degradation. Mol Microbiol. 2011;80:219–230. doi: 10.1111/j.1365-2958.2011.07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud-Sparagano MH, Maresca M, Kenny B. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell Microbiol. 2007;9:1909–1921. doi: 10.1111/j.1462-5822.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser-Silverman E, Elgrably-Weiss M, Rosenshine I, Kohen R, Altuvia S. Characterization of Escherichia coli DNA lesions generated within J774 macrophages. J Bacteriol. 2000;182:5225–5230. doi: 10.1128/jb.182.18.5225-5230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I, Gill N, Jogova M, Tam N, Robertson M, de Llanos R, et al. Salmonella SPI-1-mediated neutrophil recruitment during enteric colitis is associated with reduction and alteration in intestinal microbiota. Gut Microbes. 2010;1:30–41. doi: 10.4161/gmic.1.1.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N, Shaw R, Crepin VF, Mundy R, FitzGerald AJ, Cummings N, et al. The enteropathogenic Escherichia coli type III secretion system effector Map binds EBP50/NHERF1: implication for cell signalling and diarrhoea. Mol Microbiol. 2006;60:349–363. doi: 10.1111/j.1365-2958.2006.05109.x. [DOI] [PubMed] [Google Scholar]
- Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiya K, Nikai T. Salmonella enterica serovar Typhimurium infection induces cyclooxygenase 2 expression in macrophages: involvement of Salmonella pathogenicity island 2. Infect Immun. 2004;72:6860–6869. doi: 10.1128/IAI.72.12.6860-6869.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen MK, Peltola T, Junnila SY, Olkkonen L, Jarvinen H, Kuistila M, Huovinen P. Outbreak of diarrhoea due to Escherichia coli O111:B4 in schoolchildren and adults: association of Vi antigen-like reactivity. Lancet. 1990;336:831–834. doi: 10.1016/0140-6736(90)92337-h. [DOI] [PubMed] [Google Scholar]
- Vossenkamper A, Marches O, Fairclough PD, Warnes G, Stagg AJ, Lindsay JO, et al. Inhibition of NF-kappaB signaling in human dendritic cells by the enteropathogenic Escherichia coli effector protein NleE. J Immunol. 2010;185:4118–4127. doi: 10.4049/jimmunol.1000500. [DOI] [PubMed] [Google Scholar]
- Wan F, Weaver A, Gao X, Bern M, Hardwidge PR, Lenardo MJ. IKKbeta phosphorylation regulates RPS3 nuclear translocation and NF-kappaB function during infection with Escherichia coli strain O157:H7. Nat Immunol. 2011;12:335–343. doi: 10.1038/ni.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles S, Clare S, Harker J, Huett A, Young D, Dougan G, Frankel G. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell Microbiol. 2004;6:963–972. doi: 10.1111/j.1462-5822.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol. 2011;80:1420–1438. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- Yen H, Ooka T, Iguchi A, Hayashi T, Sugimoto N, Tobe T. NleC, a type III secretion protease, compromises NF-kappaB activation by targeting p65/RelA. PLoS Pathog. 2010;6:e1001231. doi: 10.1371/journal.ppat.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.