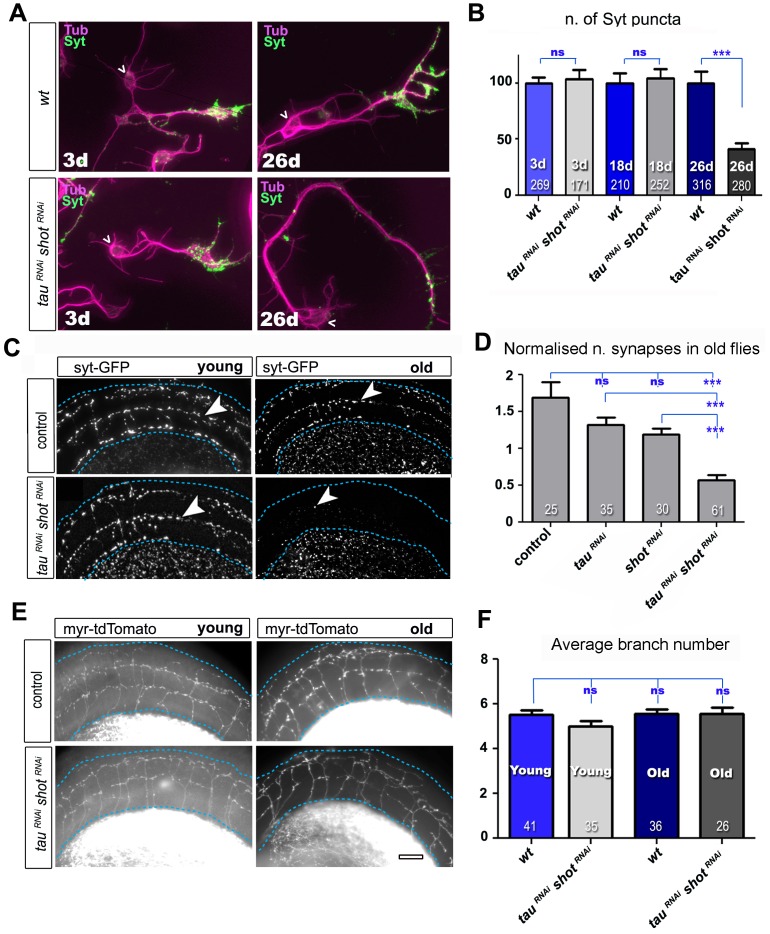
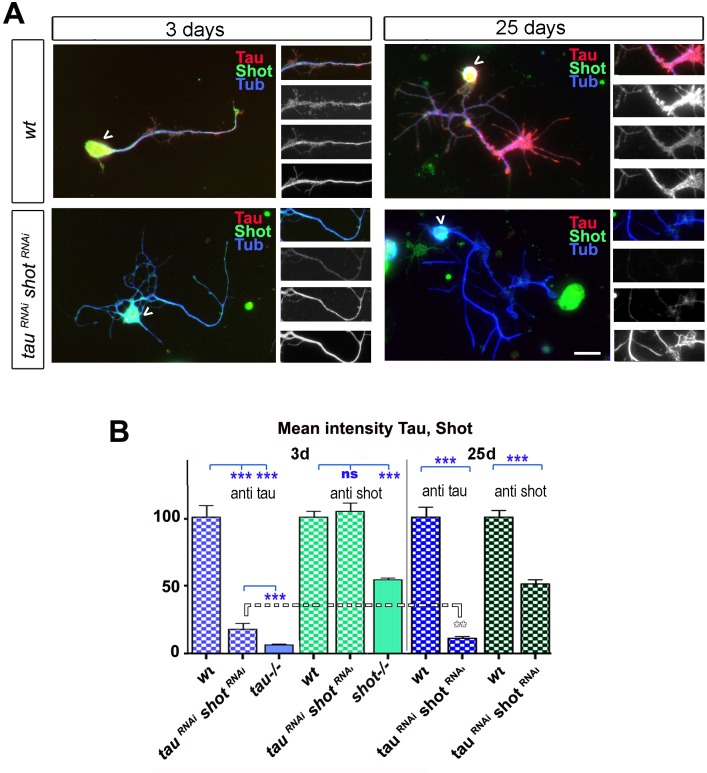
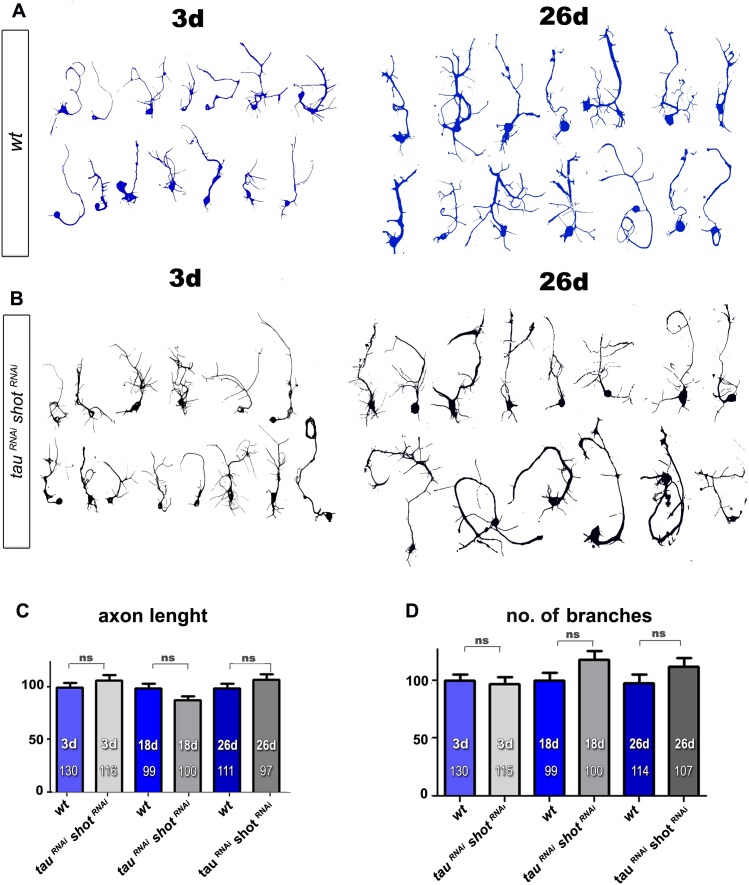
Figure 3. Tau and Shot are required for the maintenance of synaptic markers in cultured neurons and the ageing adult fly brain.
(A) Primary neurons at 3 DIV and 26 DIV cultured from embryos that were wildtype or jointly expressing UAS-tauRNAi and UAS-shotRNAi in all neurons driven by the pan-neuronal driver elav-Gal4 (tauRNAi shotRNAi). Neurons are stained with anti-tubulin and anti-Syt; at 26 DIV, tauRNAi shotRNAineurons display a reduction in the number of Syt puncta when compared to wildtype. (B) Quantification of the experiments in A, shown as the number of Syt puncta per neuron at 3 DIV, 18 DIV and 26 DIV, normalised to wildtype controls (the number of assessed neurons is indicated in each bar; ***PMW<0.001; ns, not significant PMW>0.05). (C) A region of Drosophila adult brains including the medulla (delimited by dashed lines) where Syt-GFP is expressed in dorsal cluster neurons using atonal-Gal4, in the absence (control) or together with tauRNAi and shotRNAi (tauRNAi shotRNAi). Brains are stained with anti-GFP at 2–5 days (young) and 24–29 days (old) after eclosion. Note that GFP-labelled synapses (arrowheads) are decreased in old brains upon shot and tau knock-down. (D) Quantification of the experiments in C, showing the normalised number of Syt-GFP-labelled puncta in old specimen per mean number of puncta in young specimens for the following phenotypes: ato-Gal4 UAS-syt-GFP alone (control), co-expressing UAS-tauRNAi (tauRNAi), UAS-shotRNAi(shotRNAi), or both knock-down constructs (tauRNAi shotRNAi; the number of analysed brains is indicated in each bar, ***PMW<0.001; ns, not significant PMW>0.05). (E) Brain regions as in C, of animals expressing the membrane marker myr-tdTomato driven by ato-Gal4 revealing the morphology of the projections of dorsal cluster neurons within the medulla ; brains were from adults at 2–5 days (young) and 24–29 days (old) after eclosure, expressing myr-tdTomato either alone (control) or together with tauRNAi and shotRNAi(tauRNAi shotRNAi). (F) Quantification of the experiments in E, displayed as number of branches per axon projecting into the medulla (the number of axons analysed is indicated in each bar; ns, not significant PMW>0.05). Scale bar: 10 µm in A and 40 µm in C and E. A statistics summary of the data shown here is available in Figure 3—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.14694.011