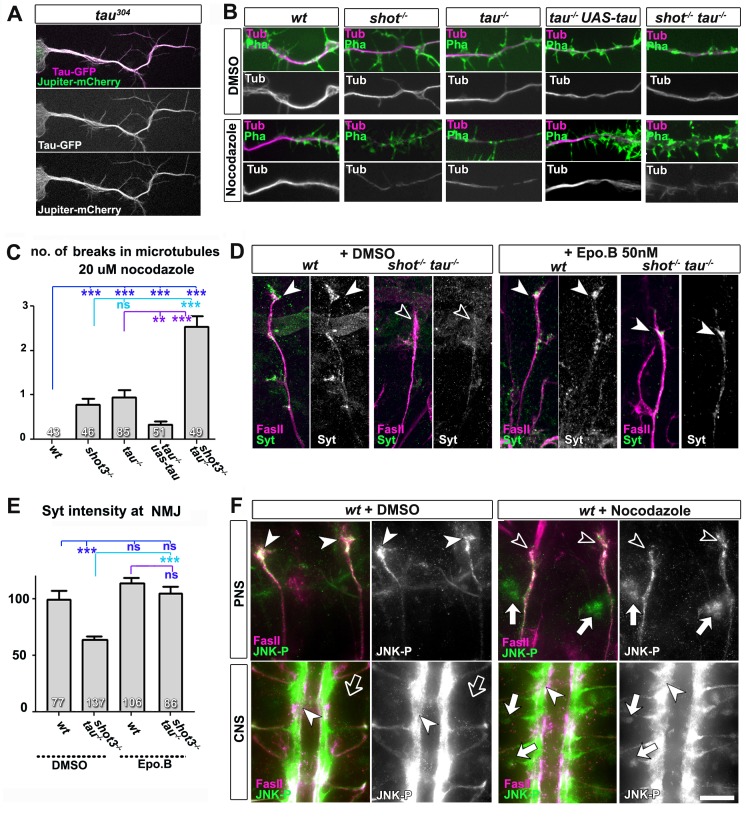
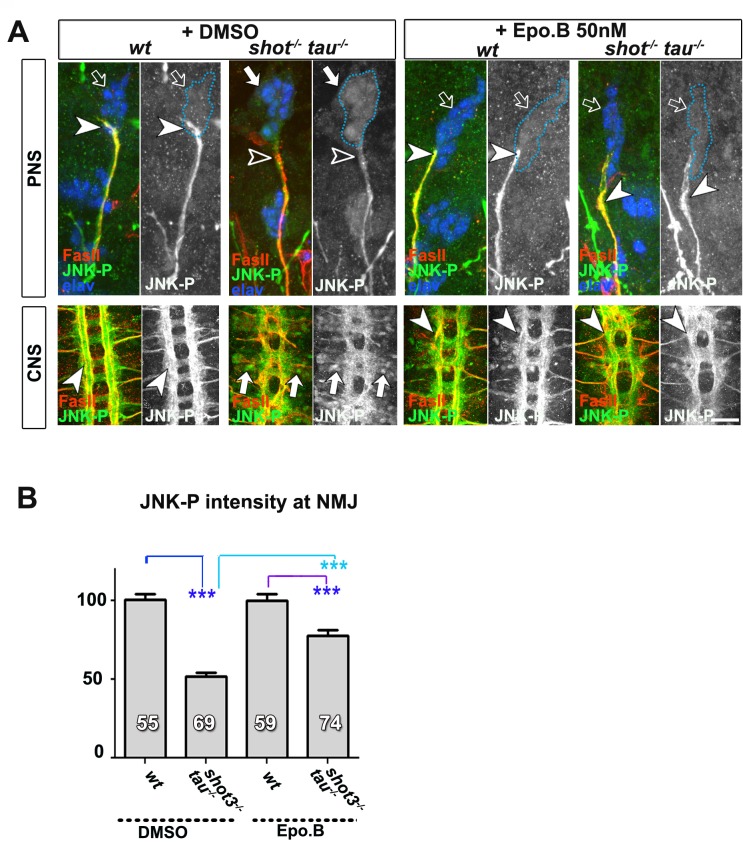
Figure 6. Microtubule instability mediates aberrant JNK signalling and synaptic defects.
(A) Live imaging of Drosophila neurons at 2 DIV, obtained from embryos carrying tau304 (a protein trap line where the endogenous tau gene is genomically tagged with GFP) and the microtubule binding protein Jupiter-Cherry. Endogenous Tau (in magenta) is observed in a pattern reminiscent of microtubules, and colocalises with Jupiter (shown in green). (B) Axons of Drosophila neurons at 6 HIV with the following genotypes: wildtype (wt), shot3 (shot-/-), tauMR22 (tau-/-), tau rescue (tau-/- UAS-tau) and shot-tau (shot-/- tau-/-). Neurons were treated for 2.5 hrs with vehicle (DMSO) or 20 µM nocodazole, fixed and stained with anti-Tubulin (Tub, magenta and white) and phalloidin (Pha, green). Only shot3, tauMR22, and shot-tau double mutant displayed gaps in their axonal microtubule bundles upon nocodazole treatment, but not wildtype and tau mutant embryos with Tau rescue. (C) Quantification of the experiments in B, indicated as the number of breaks in the microtubule staining per axon (number of analysed neurons is indicated in bars; ***PMW<0.001; **PMW<0.01; ns, not significant PMW>0.05). (D) Embryonic motoraxons of wildtype and shot-tau embryos at late stage 16 treated with vehicle (DMSO) or 50 nM of the microtubule stabilising drug epothilone B for 3 hr and stained with FasII (magenta) and Syt (green); in wildtype, the nascent NMJ at the nerve tip contains high levels of Syt (arrowheads); in shot-tau embryos there is only little Syt staining at the nerve tip (open arrowhead). Treatment of shot-tau embryos with 50nM epothilone B increases the levels of Syt at the tip of motornerves (arrowheads). (E) Quantification of the experiments shown in D, measured as the average intensity of Syt at nascent NMJs and normalised to wildtype (number or assessed NMJ is indicated in bars; ***PMW<0.001; ns, not significant PMW >0.05). (F) Upper (PNS) and lower (CNS) panels show the same locations of late stage 16 wildtype embryos as shown in Figure 5C, stained for FasII (magenta) and activated phospho-JNK (JNK-P); treatment with 100 µm nocodazole for 2 hrs induced a relocation of JNK-P from nascent NMJs (open versus white arrow heads) to cell bodies of sensory neurons and in the CNS cortex (white versus open arrows). Scale bar: 5 μm in A, 4 μm in B, 10 μm in E, 15 μm in D/PNS and 35 μm in D/CNS. A statistics summary of the data shown here is available in Figure 6—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.14694.025