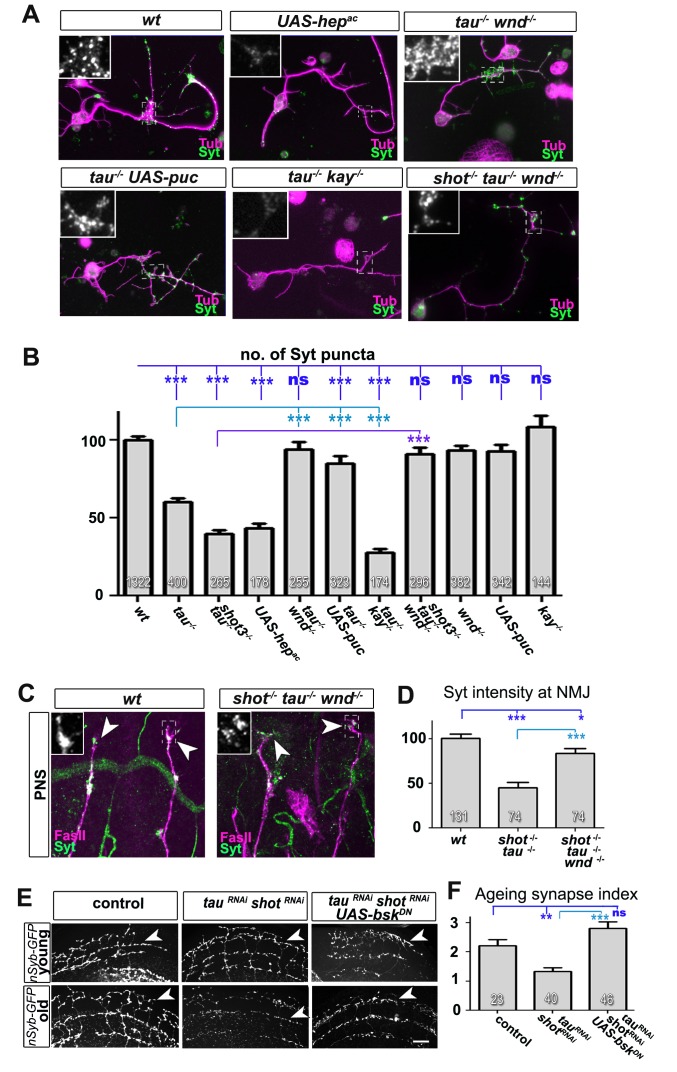
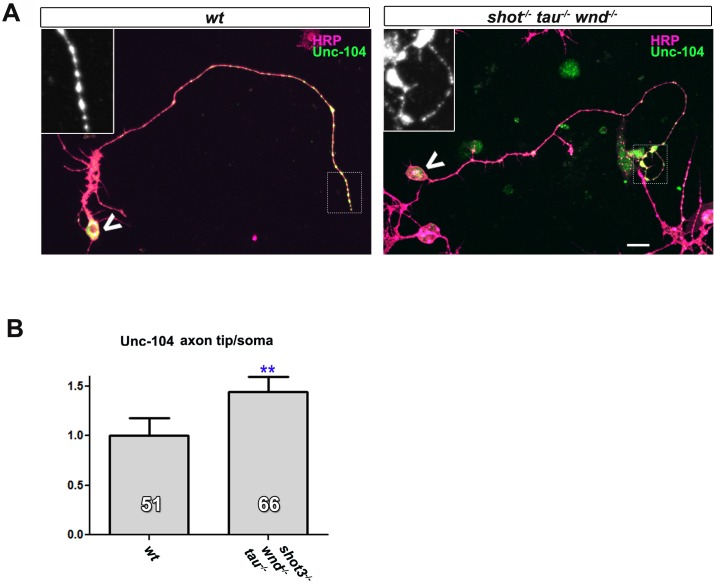
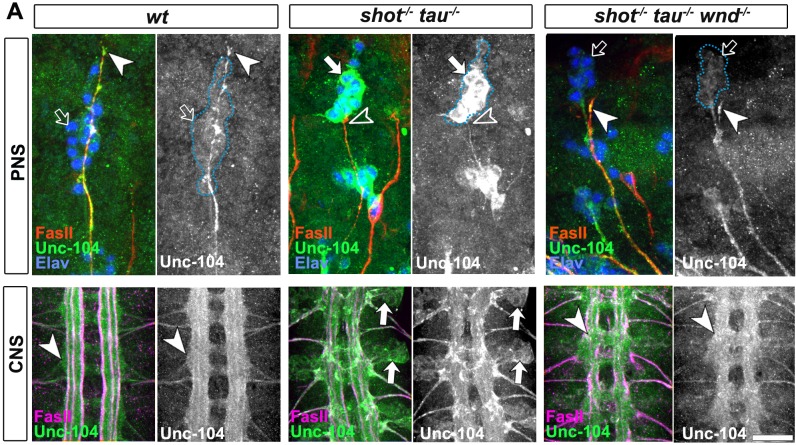
Figure 8. Inhibition of the JNK pathway rescues synaptic defects in shot-tau mutant neurons.
(A) Primary Drosophila neurons at 2 DIV, obtained from embryos of the following genotypes: wildtype (wt), elav-Gal4 driven expression of UAS-hepac (UAS-hepac), tauMR22 (tau-/-), wnd2 (wnd-/-), tau-/- with elav-Gal4 driven expression of UAS-puc (tau-/- UAS-puc), tauMR22 kay2 (tau-/- kay-/-) and shot3 tauMR22 wnd2 (shot-/- tau-/- wnd-/-), all stained with antibodies against Tubulin (tub, magenta) and Syt (green). Insets correspond to emboxed areas and show a magnified view of the Syt staining. (B) Quantification of experiments in A, shown as the number of Syt puncta normalised to wildtype (number of assessed neurons is shown in the bars, ***PMW<0.001; **PMW<0.01; *PMW<0.05; ns, not significant PMW>0.05). (C) Inter-segmental motornerves in the dorsal area of wildtype and shot3 mutant embryos at late stage 16, stained against FasII (magenta) and Syt (green); insets correspond to emboxed areas and show a magnified view of the most dorsal nascent NMJs stained for Syt; note the rescue of Syt localisation if Wnd is absent in tau-shot mutant background. (D) Quantification of the experiments in C, measured as the average intensity of Syt normalised to wt (number of assessed NMJs is shown in the bars; ***PMW<0.001; *PMW<0.01). (E) A region of Drosophila adult brains including the medulla; UAS-nSyb-GFP is expressed in dorsal cluster neurons using atonal-Gal4, either alone (control), together with tauRNAi and shotRNAi (tauRNAi shotRNAi) or together with tauRNAi, shotRNAi and UAS-bskDN. Brains are shown at 2–6 days (young) and 26–30 days at 29°C after eclosion (old); GFP-labelled synapses are decreased in old brains with shot-tau knock-down when compared to controls, and this effect is rescued by the expression of BskDN. (F) Quantification of experiments in E, shown as number of GFP-labelled synapses in old specimen per mean number of GFP-labelled synapses in young specimens of the respective genotype (number of analysed brains is indicated in the bars; ***PMW<0.001; **PMW<0.01). Scale bars: 5 μm in A, 10 μm in C and 40 μm in E. A statistics summary of the data shown here is available in Figure 8—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.14694.031