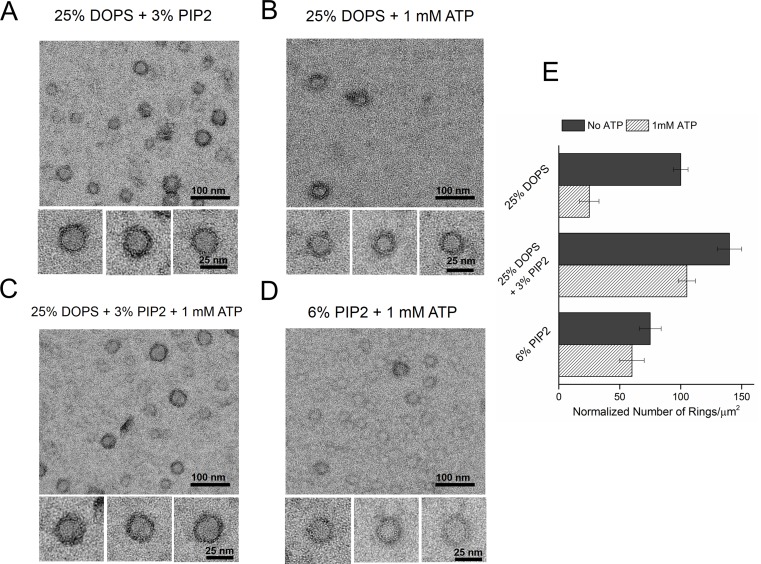
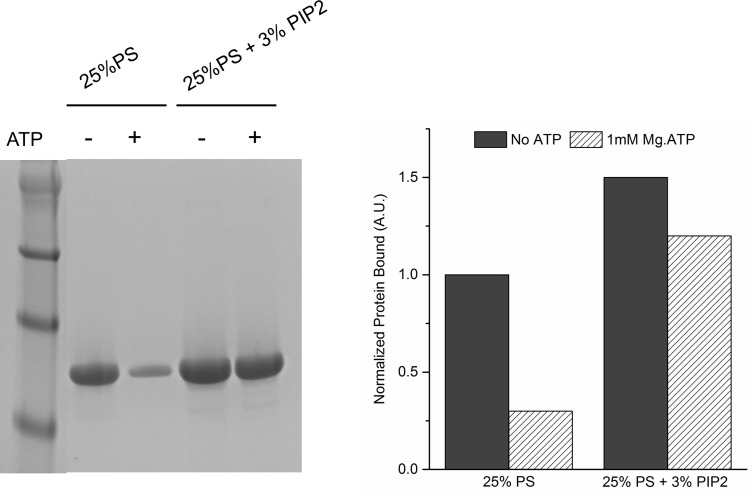
Figure 3. Syt1-PIP2 interaction is key to ring-formation under physiologically relevant conditions.
(A) Inclusion of 3% PIP2 (in addition to 25% PS) in the monolayer stabilized the ring structures and increased the number of rings observed. (B, C) Addition of ATP drastically reduced the number of rings observed in monolayers containing 25% PS only, but not when supplemented with 3% PIP2. (D) PIP2 (6%) as the only anionic lipid on the bilayer was sufficient to assemble ring-like oligomers, even in the presence of 1 mM ATP. All EM analyses were carried out using 5 µM protein in buffer containing 100 mM KCl and 1 mM free Mg2+. Representative micrographs and average values/SEM from a minimum of three independent trials are shown in (E). The rings observed under all conditions shown in (E) were similarly (~30 nm) sized